Ipconazole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| View without stereoisomers | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ipconazole | ||||||||||||||||||
| other names |
(1 RS , 2 SR , 5 RS ; 1 RS , 2 SR , 5 SR ) -2- (4-chlorobenzyl) -5-isopropyl-1- (1 H -1,2,4-triazol-1-ylmethyl) cyclopentanol |
||||||||||||||||||
| Molecular formula | C 18 H 24 ClN 3 O | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 333.861 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.2 g cm −3 |
||||||||||||||||||
| Melting point |
88-90 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water (20 mg l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ipconazole is a mixture of several stereoisomeric chemical compounds from the group of triazoles . It is used as a plant protection product.
Extraction and presentation
Ipconazole can be obtained from 2-methoxycarbonylcyclopentanone through a multistage reaction.
Stereochemistry
When considering the stereoisomers of ipconazole, the following numbering of the stereocenters on the cyclopentane ring is used:
- a hydroxyl group is attached to position 1 ,
- a p -chlorobenzyl radical is attached to position 2 and
- an isopropyl group is attached to position 5 .
Applied to the table below, the result is that the racemate in the first row consists of the (1 S , 2 R , 5 S ) isomer and the (1 R , 2 S , 5 R ) isomer. The nomenclature of the other stereoisomers follows the same principle.
Generally speaking, chemical compounds with multiple stereocenters form up to 2 n stereoisomers. Here n is the number of stereocenters. Accordingly, there are 8 stereoisomers in Ipconazole, which have also been confirmed experimentally.
According to the Federal Office for Consumer Protection and Food Safety , the technical active ingredient that is used as a fungicide contains four stereoisomers. These are the two racemates that are shown in the table in the top two lines:
| Four racemic pairs of diastereomers | |
|---|---|
  CAS number: 115850-69-6 |
|
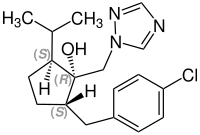  CAS number: 115937-89-8 |
|
  CAS number: 115937-88-7 |
|
  CAS number: 115937-91-2 |
properties
Ipconazole is a colorless solid that is practically insoluble in water. There is a 9: 1 cis - cis - ,: cis -, trans - stereoisomers ago.
use
Ipconazole is used as a fungicide . It was developed by Kureha and launched in 1993. It works by inhibiting the sterol - biosynthesis by demethylation at carbon atom C14 of the steroid -Gerüsts inhibits. It is used against a wide range of fungal diseases, especially in rice, but also in other crops.
Admission
From September 1, 2014, the connection will be approved in the EU. In Switzerland there are currently none, in Germany, Austria and other EU countries pesticides with this active ingredient are approved.
safety instructions
Ipconazole is on the Greenpeace blacklist of pesticides due to its toxicity.
Individual evidence
- ↑ a b c d e f g Entry on Ipconazole. In: Römpp Online . Georg Thieme Verlag, accessed on January 22, 2015.
- ↑ a b US EPA - Pesticides - Fact Sheet: Ipconazole , accessed January 22, 2015.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel (eds.): Modern Crop Protection Compounds. Volume 1: Herbicides. 2nd, revised and enlarged edition. Wiley-VCH, Weinheim 2012, p. 785, ( limited preview in the Google book search).
- ↑ Paula Y. Bruice: Organic Chemistry: Study compact . Pearson Studium, Munich 2011, ISBN 978-3-86894-102-9 , p. 205.
- ↑ Elim M. Ulrich, Candice M. Morrison, Michael M. Goldsmith, William T. Foremann: Chiral Pesticides: Identification, Description, and Environmental Implications . In: Reviews of Environmental Contaminations and Toxicology. Springer 2012, Boston, Volume 217, pp. 1–74, DOI: 10.1007 / 978-1-4014-2329-4_1 , see p. 10.
- ↑ Pesticides with isomer peaks when determined with GC and HPLC . (PDF) Federal Office for Consumer Protection and Food Safety, accessed on January 25, 2018 .
- ↑ Implementing Regulation (EU) No. 571/2014 of the Commission of May 26, 2014 approving the active substance ipconazole according to Regulation (EC) No. 1107/2009 of the European Parliament and of the Council on the placing of plant protection products on the market and amending the annex to the Commission Implementing Regulation (EU) No. 540/2011 , accessed on January 22, 2015
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Ipconazole in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 6, 2019.
- ↑ Lars Neumeister, with contribution from Wolfgang Reuther Design Monika Sigmund Photos front cover: Philip Reynaers, page 2-3: Bernhard Nimtsch, Juergen Siegmann, Bob Edwards, Juergen Siegmann, Ángel Garcia: The EU Pesticide Blacklist. In: Greenpeace. Greenpeace, accessed February 27, 2020 .
