Isofenphos
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
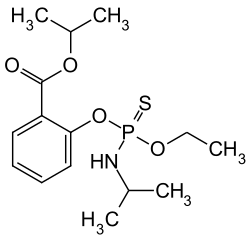
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isofenphos | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 15 H 24 NO 4 PS | |||||||||||||||
| Brief description |
yellow to brown flammable liquid with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 345.4 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.133 g cm −3 |
|||||||||||||||
| Melting point |
−21 ° C |
|||||||||||||||
| boiling point |
250 ° C |
|||||||||||||||
| Vapor pressure |
0.0000044 hPa (25 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Isofenphos is an insecticide that was previously used for seed dressing . Isofenphos is one of the phosphoric acid esters . In tropical countries it was also used to control termites.
Extraction
Isofenphos is made from phosphorus trichloride . This reacts with sulfur in the presence of aluminum chloride to form thiophosphoryl trichloride , which reacts with ethanol to form ethylthiophosphorus dichloride . The next steps are the reactions with isopropyl salicylate and the subsequent intermediate with isopropylamine .
Admission
The EU Commission decided in 2002 not to include isofenphos in the list of permitted active ingredients in pesticides . It is no longer contained in plant protection products that are approved in Germany, Austria or Switzerland.
Individual evidence
- ↑ a b c d e f g h i j k Entry on isofenphos in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ SD Gangolli, Royal Society of Chemistry (Great Britain): The Dictionary of Substances and Their Effects (DOSE): Volume 04 EJ . Royal Society of Chemistry, 1999, ISBN 0-85404-823-5 , pp. 840 ( limited preview in Google Book search).
- ↑ Entry on Isofenphos in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 378 ( limited preview in Google Book search).
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 extending the deadline in accordance with Article 8 paragraph 2 of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this directive and the revocation of Approvals of plant protection products with these active ingredients (PDF) .
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on isofenphos in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved February 19, 2016.


