Potassium hexafluorosilicate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
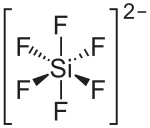
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium hexafluorosilicate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | K 2 [SiF 6 ] | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 220.27 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.27 g cm −3 (other source 2.71 g cm −3 ) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium hexafluorosilicate is an inorganic chemical compound of potassium from the group of hexafluorosilicates and the potassium salt of hexafluorosilicic acid .
Occurrence
Potassium hexafluorosilicate occurs naturally in the form of the minerals hieratite and demartinite .
Extraction and presentation
Potassium hexafluorosilicate can be obtained by reacting hexafluoridosilicic acid with potassium hydroxide or potassium chloride .
properties
Potassium hexafluorosilicate is a white solid that is sparingly soluble in water. It decomposes when heated or in hot water, producing hydrogen fluoride and silicon tetrafluoride . It can be melted without decomposition with the addition of potassium fluoride . It has a cubic crystal structure with the space group Fm 3 m (space group no. 225) . A second modification with the space group P 6 3 mc (space group no.186) is also known.
use
Potassium hexafluorosilicate is used in the manufacture of porcelain and also for the preservation of wood. It is involved in the manufacture of ceramics , aluminum and magnesium melts and also serves as an intermediate product in the manufacture of optical glass.
Individual evidence
- ↑ a b c d e f g h Entry on potassium hexafluorosilicate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b J. H. Loehlin: Redetermination of the structure of potassium hexafluorosilicate, K 2 SiF 6 . In: Acta Crystallographica Section C Crystal Structure Communications . tape 40 , no. 3 , March 15, 1984, p. 570-570 , doi : 10.1107 / s0108270184004893 .
- ^ A b R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 524 ( limited preview in Google Book search).
- ^ Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . CRC Press, 2016, ISBN 978-1-4398-1462-8 , pp. 324 ( limited preview in Google Book search).
- ↑ Entry on alkali hexafluorosilicates (K) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 21, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ mineralienatlas.de: Mineralienatlas Lexikon - Hieratit , accessed on November 21, 2016
- ↑ Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 978-0-412-30120-9 , pp. 3217 ( limited preview in Google Book search).
- ↑ D. Menz, W. Wilde, L. Kolditz, K. Heide: Dynamic thermal analysis of the decomposition of K 2 SiF 6 . In: Journal of Fluorine Chemistry . 24 (3), 1984, pp. 345-354, doi : 10.1016 / S0022-1139 (00) 81323-8 .
- ↑ Data sheet Potassium hexafluorosilicate, 99.999% (metals basis) from AlfaAesar, accessed on November 21, 2016 ( PDF )(JavaScript required) .

![{\ displaystyle \ mathrm {2 \ KCl + H_ {2} [SiF_ {6}] \ longrightarrow K_ {2} [SiF_ {6}] + 2 \ HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7eda1bd53038b26e50362d745cc445f26b9d9e8b)