Potassium tetrafluoroborate
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
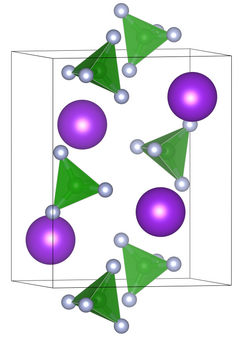
| __ K + __ B 3+ __ F - | ||||||||||||||||
| Crystal system |
orthorhombic |
|||||||||||||||
| Space group |
Pbnm (No. 62, position 3) |
|||||||||||||||
| Lattice parameters |
a = 7.03 Å , b = 8.674 Å, c = 5.496 Å |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium tetrafluoroborate | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | K [BF 4 ] | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 125.91 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.5 g cm −3 |
|||||||||||||||
| Melting point |
530 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in water (4.4 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium tetrafluoroborate is a chemical compound , more precisely the potassium salt of tetrafluoroboric acid . The correct scientific name for the latest IUPAC - nomenclature is tetrafluoridoborat potassium.
Extraction and presentation
Potassium tetrafluoroborate can be obtained by reaction or neutralization of potassium hydroxide or potassium carbonate with tetrafluoridoboric acid.
properties
Potassium tetrafluoroborate is a white, odorless solid that is sparingly soluble in water. It decomposes at a temperature above 530 ° C, hydrogen fluoride , boron oxide , potassium oxide and boron fluoride arise. The compound crystallizes in the orthorhombic space group Pbnm (space group no. 62, position 3) with the lattice constants a = 7.03 Å , b = 8.674 Å and c = 5.496 Å. There are four formula units in a unit cell.
use
Potassium tetrafluoroborate is used as solder , cover salt when melting light metal , molding sands when melting aluminum and manganese and is contained in phosphating baths.
Individual evidence
- ↑ a b M. JR Clark, H. Lynton: Crystal strucutes of potassium, ammonium, rubidium and cesium tetrafluoroborates . In: Canadian Journal of Chemistry . tape 47 , no. 14 , January 9, 1969, p. 2579-2586 , doi : 10.1139 / v69-426 .
- ↑ a b c d e f g h Entry for CAS no. 14075-53-7 in the GESTIS substance database of the IFA , accessed on February 17, 2011 (JavaScript required)
- ↑ Data sheet potassium tetrafluoroborate from Sigma-Aldrich , accessed on February 17, 2011 ( PDF ).
- ↑ Toxicological evaluation of tetrafluoroboric acid and salts (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
![{\ displaystyle {\ ce {KOH + HBF4 -> K [BF_4] + H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2759f3fb65fa04504b35b134d6dc658e8dd3d877)
![{\ displaystyle {\ ce {K2CO3 + 2HBF4 -> 2K [BF4] + H2O + CO2 ^}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0da5151339de96f06c0d7598382a83774dbed9f4)