Bone density
Under the bone mineral density (also bone mineral density , English density Bone, bone mineral density (BMD) ) is defined as the ratio of mineralized bone to a defined volume of bone.
Basics
The bone consists of a hard layer ( substantia corticalis ) and a mineral layer ( substantia spongiosa ). The latter is formed by the incorporation of calcium phosphate in the form of incompletely crystalline hydroxyapatite in the organic bone matrix . Incompletely crystalline carbonate apatite [Ca 10 (PO 4 ) 6 (OH) 2 ] or bone apatite is the main mineral component of bone. One of the structures that make up the mineral layer of bone, which resembles tubes, is called the Haversian Canal . This structure serves, among other things, for the supply of nutrients that the bone system needs. Thin plates called lamellae , which contain the bone marrow of the bone, surround the Haversian Canal. In the yellow bone marrow (fat marrow, Latin medulla ossium flava ), particularly large amounts of fat are stored in the reticular cells . The blood-forming cells are found in the red bone marrow ( Latin: Medulla ossium rubra ). The hard bone layer is made up of collagen . This layer makes up 70% of the bone density in adults and 30% in children. Three types of cells make up the functional part of bone, the osteoblasts , osteocytes and osteoclasts . Howship lacunae arise from the resorption of bone substance on the surfaces of the trabeculae (bone trabeculae) of the substantia spongiosa . They are signs of the ongoing bone remodeling. Active osteoclast groups eat their way through the bone where remodeling is necessary. New bone lamellae are formed by osteoblasts. The result of this process is called bone turnover ( English Bone turnover referred). The osteocytes control the mineral balance in the body.
Influences on bone density
Bone tissue is a very active tissue that is constantly being built up and broken down, meaning that 20 to 40 percent of the skeleton is renewed every year . Bone mass increases with growing age and reaches its peak at around 20 years ( English Peak Bone Mass , pbm). Bone density is subject to numerous hormonal influences, including the growth hormone somatropin , sex hormones and steroid hormones . The two main mechanical functions of the skeleton are to give the muscles a lever to develop force and to support the body against gravity . Bone growth and bone resorption are determined by the maximum elastic deformation of the bone, which the Mechanostat model describes. In order to fulfill these functions permanently, the skeleton has to constantly adapt its structure to new mechanical requirements in accordance with Wolff's law . These adaptation processes are called mechanotransduction , with which the bone density changes in the individual bones. Mechanical signals are converted into cellular signals. The shear forces caused by mechanical stimulation are perceived by the osteocytes and lead to a modulation of the release of various mediators that are essential for the balance of bone metabolism . The result is an increase in osteoblast activity and an inhibition of osteoclast activity.
A normal bone density is 150 mg / ml with a standard deviation of 20 mg / ml for both sexes and regardless of age until the onset of puberty .
The trabecular peak bone mass in the lumbar spine is higher in male subjects than in female subjects ; the bone density at the time of peak bone mass is higher in female subjects than in male subjects.
Development of bone density
Aged between 20 and 30 years, the holding bone loss or less balanced with the bone structure. After that, the breakdown predominates, with the physiological bone loss being around 0.3 to 0.5 percent of the bone mass per year. At the age of 50 you already have 6–10 percent less bone mass, making the skeleton more prone to breakage. After menopause (menopause), women lose 1 to 2 percent of their bone density every year with a drop in estrogen levels , which can lead to osteopenia or osteoporosis . This bone loss is obviously genetically programmed. As a natural suppressor of the protein Receptor Activator of NF-κB Ligand (RANKL), estrogen is no longer available in sufficient quantities. RANKL is significantly involved in the regulation of bone remodeling , especially osteoclast differentiation , which reduces bone density.
Bone Density in Astronauts

During a space flight, space fliers develop a continuously progressive negative calcium balance due to weightlessness . The calcium loss of the Skylab crew reached about 300 mg / d on flight day 84. The resulting mean loss of bone density ( vertebrae , femoral neck , trochanter , pelvic bones ) was 1% to 1.6% per month, despite the extreme training program on load-bearing bones, but with large individual differences. After the flight, the bone density recovered in the long term. After a 30-month flight to Mars , pronounced osteoporosis would be threatened.
Bone density in implants
A strong periprosthetic loss of bone density occurs within the first 3 months after implantation of a hip endoprosthesis . This rapid loss of bone density is also a result of the initial surgical irritation and the immobilization of the patient. It is also caused by the changed flow of forces with stress shielding as well as relieving and reducing the load on the operated extremity . According to Wolff's law , a bone will always adapt to its function and degenerate under low loads. Particularly noticeable is the considerable decrease in bone density in the proximal part of the femur in the region of the calcar femoris (thickening in the dorsal half of the proximal femur). After the initial remodeling process , which lasts 12 months to 2 years, no relevant loss of bone density can be detected and a so-called steady state of bone density is established.
T-Score and Z-Score
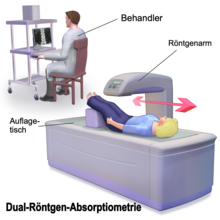
The bone density is determined by means of bone density measurement (osteodensitometry). Bone mineral content is measured ( English Bone mineral content , bone mineral density) in grams and ( English Bone mineral density ) in g / cm² or g / cc, depending on the process of bone densitometry. The measured value does not provide any information about the three-dimensional geometry of the measuring object and thus no density values in the physical sense ( SI unit of density: g / cm³), but a surface-projected mass (SI unit: g / cm²), also known as area density .
With the established survey methods include dual-energy x-ray absorptiometry ( English dual-energy X-ray absorptiometry , DXA, DEXA) and quantitative computed tomography (QCT). The T-Score indicates the standard deviation of the age-dependent mean value of the peak bone mass (normal value T> -1.0). A so-called Z-Score is also given, which relates to healthy men or women of the same age. A normal Z-Score (> -1) indicates that the bone density is age-typical.
- A T-score of ≥ -1 is considered normal.
- With a T-score between −1 and −2.5, there is no osteoporosis, but a preliminary stage, the so-called osteopenia.
- A T-score of <−2.5 leads to the diagnosis of osteoporosis .
In addition to the pure measured value, factors such as age and gender are decisive.
Pathological changes in bone density
The bone density can be generalized or circumscribed, decreased or increased.
- There is decreased bone density
- There is increased bone density
Web links
- Guideline Osteoporosis 2017 , AWMV 183/001, umbrella organization of the German-speaking scientific osteological societies . Retrieved February 10, 2019.
Individual evidence
- ^ Jörg Jerosch, Augustinus Bader, Günter clock: Bones: Curasan pocket atlas special . Georg Thieme Verlag, 2002, ISBN 978-3-13-132921-9 , p. 34.
- ↑ Kathy Donina, Density Of Bone , The Physics Factbook, 2002. Retrieved February 13, 2018.
- ↑ K. Kerschan-Schindl, The Mechanostat Model , J Miner Metabolism 2012; 19 (4), pp. 159-62.
- ^ RL Duncan, CH Turner: Mechanotransduction and the functional response of bone to mechanical strain. In: Calcified tissue international. Volume 57, Number 5, November 1995, ISSN 0171-967X , pp. 344-358, PMID 8564797 (review).
- ↑ L. Berthold, G. Haras, M. Mann, G. Alzen: Trabecular bone density of the lumbar spine in children and adolescents in quantitative CT: reference values and peak bone mass. In: RöFo - Advances in the field of X-rays and imaging processes. 178, 2006, p. 1235, doi : 10.1055 / s-2006-927151 .
- ↑ Peter Echevers H .: Vitamin D: The Pharma Scandal . LULU Press Enterprises, September 9, 2013, p. 64. ISBN 978-1-291-52930-2
- ↑ Michael McClung: Role of RANKL inhibition in osteoporosis. In: Arthritis Research & Therapy. 9, p. S3, doi : 10.1186 / ar2167 .
- ^ A. LeBlanc, L. Shackelford, V. Schneider: Future Human Bone Research in Space . In: Bone . Volume 22, Number 5, Supp. 1, May 1998, pp. 113-116, doi : 10.1016 / S8756-3282 (98) 00013-1 .
- ^ Jörg Jerosch, Augustinus Bader, Günter clock: Bones: Curasan pocket atlas special . Georg Thieme Verlag, 2002, ISBN 978-3-13-132921-9 , p. 56.
- ^ JC Buckey: Preparing for Mars: the physiologic and medical challenges. In: European journal of medical research. Volume 4, Number 9, September 1999, pp. 353-356, PMID 10477498 (review).
- ^ A. Hawkey: Physiological and biomechanical considerations for a human Mars mission. In: Journal of the British Interplanetary Society. Volume 58, Numbers 3-4, 2005 Mar-Apr, pp 117-130, PMID 15852539 .
- ↑ M. Niinomi, M. Nakai: Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. In: International Journal of Biomaterials. 2011, p. 1, doi : 10.1155 / 2011/836587 .
- ↑ Klaus M. Peters, Dietmar Pierre König: Advanced Training in Osteology 2 . Springer-Verlag, September 2, 2008, ISBN 978-3-7985-1825-4 , p. 70.
- ↑ Reiner Bartl: Osteoporosis: Prevention - Diagnostics - Therapy; 9 tables . Georg Thieme Verlag, 2004, ISBN 978-3-13-105752-5 , p. 46.
- ↑ Matthias Angstwurm, Thomas Kia: mediscript StaR 1 the state examination revision course for cardiology and angiology . Elsevier, Urban & Fischer Verlag, November 22, 2012, ISBN 978-3-437-29441-9 , p. 46.
- ↑ F. Hefti: Pediatric Orthopedics in Practice . Springer-Verlag, July 1, 2013, ISBN 978-3-662-08078-8 . , P. 648.