Copper (II) acetylacetonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
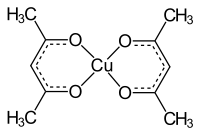
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Copper (II) acetylacetonate | |||||||||||||||
| other names |
Copper (II) -4-oxopent-2-en-2-olate |
|||||||||||||||
| Molecular formula | C 10 H 14 CuO 4 | |||||||||||||||
| Brief description |
flammable, blue-gray, odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 261.77 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
245 ° C (decomposition, from 160 ° C sublimation) |
|||||||||||||||
| Vapor pressure |
13 Pa (163 ° C) |
|||||||||||||||
| solubility |
very bad in water (0.2 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Copper (II) acetylacetonate [Cu (acac) 2 ] is a chemical compound from the group of enolates ( salts of keto-enol tautomers ) and organic copper compounds . It is a complex compound , wherein the acetylacetonate anion by binding any oxygen -atom with the copper - cation a chelate forming ring.
Extraction and presentation
Copper (II) acetylacetonate can be obtained by reacting copper sulphate with acetylacetone and sodium hydroxide solution .
use
Copper (II) acetylacetonate is used as a catalyst , stabilizer , additive and as an intermediate in the manufacture of fungicides .
Related links
- Aluminum (III) acetylacetonate
- Cobalt (III) acetylacetonate
- Copper (I) acetylacetonate
- Manganese (III) acetylacetonate
- Nickel (II) acetylacetonate
Individual evidence
- ↑ a b c d e Entry on copper (II) acetylacetonate in the GESTIS substance database of the IFA , accessed on February 20, 2017(JavaScript required) .
- ↑ Entry on copper (II) acetylacetonate at ChemBlink , accessed on February 25, 2011.
- ↑ Data sheet copper (II) acetylacetonate (PDF) from Merck , accessed on June 15, 2017.
- ↑ Antonio Carlos Burtoloso: Copper (II) acetylacetonates: An Inexpensive Multifunctional Catalyst . In: Synlett . No. 18 , 2005, ISSN 0936-5214 , p. 2859-2860 , doi : 10.1055 / s-2005-918920 .
- ↑ Raj K. Bansal: Heterocyclic Chemistry . New Age International, 1999, ISBN 81-224-1212-2 , pp. 18 ( limited preview in Google Book search).
- ↑ Peter I. Dalko: Enantioselective Organocatalysis: Reactions and Experimental Procedures . John Wiley and Sons, 2007, ISBN 3-527-31522-5 , pp. 361 ( limited preview in Google Book search).
- ↑ Janine Cossy, Stellios Arseniyadis: Modern Tools for the Synthesis of Bioactive Molecules Complex . John Wiley & Sons, 2012, ISBN 1-118-34285-2 , pp. 473 ( limited preview in Google Book search).
