Magnesium hexafluorosilicate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
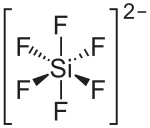
|
||||||||||
| General | ||||||||||
| Surname | Magnesium hexafluorosilicate | |||||||||
| Molecular formula | MgSiF 6 | |||||||||
| Brief description |
white odorless solid (hexahydrate) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 166.38 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
1.79 g cm −3 (hexahydrate) |
|||||||||
| Melting point |
120 ° C (decomposition) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data |
200 mg · kg -1 ( LD 50 , guinea pigs , oral ) |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Magnesium hexafluorosilicate is an inorganic chemical compound of magnesium from the group of hexafluorosilicates .
Extraction and presentation
Magnesium hexafluorosilicate can be obtained by reacting magnesium compounds with hydrofluoric acid in the presence of silicon dioxide .
properties
Magnesium hexafluorosilicate, as a hexahydrate, is a crystalline white odorless solid that is soluble in water. It has a monoclinic crystal structure with the space group P 2 1 / a (space group no. 14, position 3) . However, other phases are also possible.
use
Magnesium hexafluorosilicate is used as a hardener for concrete and as a water protection chemical. It is also used as an insecticidal wood preservative in building construction.
Individual evidence
- ↑ a b c d data sheet Magnesium hexafluorosilicate hexahydrate, 98% from AlfaAesar, accessed on November 23, 2016 ( PDF )(JavaScript required) .
- ↑ a b c d R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 562 ( limited preview in Google Book search).
- ↑ Entry on magnesium hexafluorosilicate in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ↑ Entry on magnesium hexafluorosilicate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 27, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Magnesium hexafluorosilicate from Sigma-Aldrich , accessed on November 23, 2016 ( PDF ).
- ↑ Michael Ash, Irene Ash: Handbook of Preservatives . Synapse Info Resources, 2004, ISBN 978-1-890595-66-1 , pp. 843 ( limited preview in Google Book search).
- ↑ Federal Environment Agency: Leaflet on the best available techniques for the production of bulk inorganic chemicals: ammonia, acids and fertilizers, August 2007
- ↑ Peter G. Skrylnik, Albert M. Ziatdinov: Incommensurate Phases of the MgSiF6-6H2O Crystals: EPR and Group-Theoretical Studies. In: Applied Magnetic Resonance. 45, 2014, p. 623, doi : 10.1007 / s00723-014-0542-6 .
- ↑ R. Hrabański, V. Kapustianik, V. Kardash, S. Sveleba: Birefringent and piezooptic properties of (MgSiF6) - 6H2O crystals. In: Physica Status Solidi. 142, 1994, p. 509, doi : 10.1002 / pssa.2211420225 .
- ↑ S. Syoyama, K. Osaki: An X-ray study of the low-temperature form of MgSiF6.6H2O and the relation between the crystal lattices of low- and high-temperature forms. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 28, p. 2626, doi : 10.1107 / S0567740872006624 .
- ↑ Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens . William Andrew, 2011, ISBN 978-1-4377-7869-4 , pp. 3039 ( limited preview in Google Book search).
- ↑ Ralf Steudel : Chemistry of Non-Metals From Structure and Bond to Application . Walter de Gruyter, 2008, ISBN 978-3-11-021128-3 , p. 282 ( limited preview in Google Book search).


![{\ displaystyle \ mathrm {MgO + SiO_ {2} +6 \ HF \ longrightarrow Mg [SiF_ {6}] + 3 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/897f0e068141bab15026c5edc572e5eb46a0d0f5)