Meprobamate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
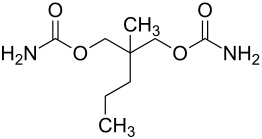
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Meprobamate | ||||||||||||||||||
| other names |
2-methyl-2-propyltrimethylene dicarbamate ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 9 H 18 N 2 O 4 | ||||||||||||||||||
| Brief description |
white to almost white, amorphous or crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 218.25 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
103-107 ° C |
||||||||||||||||||
| solubility |
sparingly soluble in water, easily soluble in ethanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Meprobamate is the active ingredient in a sedative that was launched in the United States in 1955 under the name Miltown . It belongs to the chemical compound class of urethanes and quickly became one of the best-selling drugs. It has been withdrawn from the market in Germany, Austria and Switzerland or is not prescribable.
history
Meprobamat was discovered in 1954 by Frank Milan Berger (1913–2008).
In the early 1960s, meprobamate was replaced by benzodiazepines such as chlordiazepoxide and diazepam .
Meprobamat is a marketable narcotic drug in the Federal Republic of Germany due to its listing in Appendix 2 BtMG . Handling without permission is generally a criminal offense. The prohibition does not apply to drugs that contain up to 500 mg of meprobamate per divided form without another substance from Annexes I-III. Further information can be found in the main article Narcotics Law in Germany .
In Austria, the preparation has already been withdrawn from the market due to its high addiction potential; on January 20, 2012, the European Medicines Agency also recommended such a measure.
In Switzerland, Meprobamat was on the market until October 2012 under the name Meprodil (prescription only). It was withdrawn from the market due to a reassessment of the risk-benefit ratio.
Side effects
The side effects include a. a gynecomastia .
Trade names
Microbamat (A), Meprodil (CH), Miltaun (A), Tonamyl (D)
Web links
Individual evidence
- ↑ a b c d data sheet MEPROBAMATE CRS (PDF) at EDQM , accessed on August 21, 2009.
- ↑ a b Meprobamate data sheet at Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ^ Möller, Laux, Deister and Scharm - Psychiatrie und Psychotherapie, 4th edition 2009, p. 14, ISBN 978-3-13-128544-7 .
- ↑ Entry on Meprobamat. In: Römpp Online . Georg Thieme Verlag, accessed on January 26, 2019.
- ↑ Pharma Information, Independent Information for Doctors, Volume 27 / No. 1, March 2012 Innsbruck.
- ↑ DHPC - Meprodil (Meprobamat): reassessment of the risk-benefit ratio and withdrawal from the market on October 31, 2012. (No longer available online.) Swissmedic , September 28, 2012, archived from the original on March 2, 2017 ; accessed on March 1, 2017 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.