Methacrylic acid ethyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
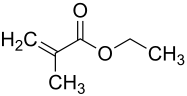
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methacrylic acid ethyl ester | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 O 2 | |||||||||||||||
| Brief description |
Highly flammable, volatile, colorless liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.91 g cm −3 |
|||||||||||||||
| Melting point |
−61 to −59 ° C |
|||||||||||||||
| boiling point |
117 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4128 (20 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethacrylic acid ethyl ester is a chemical compound from the group of carboxylic acid esters and the ethyl ester of methacrylic acid and thus an analogue of the acrylic acid ester ethyl acrylate .
properties
Methacrylic acid ethyl ester is a highly flammable, volatile, colorless liquid with a characteristic odor. The flash point is 19 ° C, the ignition temperature 385 ° C. The vapors of ethyl methacrylate can form an explosive mixture with air, which is below the explosion limit at 1.4 vol .-%. Its viscosity is 0.62 mPas. The heat of polymerization is −59.5 kJ mol −1 or −521 kJ kg −1 . It can polymerize explosively (which is why it is marketed in a stabilized form) and is sensitive to light and air.
use
Methacrylic acid ethyl ester is used for the production of acrylic polymers and plastic additives .
Risk assessment
Methacrylic acid ethyl ester was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of the substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of ethyl methacrylate were concerns about consumer use , high (aggregated) tonnage and widespread use as well as the dangers arising from a possible assignment to the group of CMR substances and the suspected dangers due to sensitizing properties. The re-evaluation took place from 2014 and was carried out by Italy . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on ethyl methacrylate in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ↑ a b c d Data sheet ethyl methacrylate (PDF) from Merck , accessed on June 12, 2010.
- ↑ Entry on ethyl methacrylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ Brandrup, J .; Immergut, EH; Grulke, EA; Abe, A .; Bloch, DR: Polymer Handbook , 4th Edition, Wiley-VCH 2003, ISBN 978-0-471-47936-9 , p. II / 369.
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Ethyl methacrylate , accessed on May 1, 2020.

