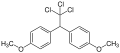
Methoxychlor
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Structural formula of p , p ′ -methoxychlor | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methoxychlor | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 15 Cl 3 O 2 | ||||||||||||||||||
| Brief description |
colorless to yellowish solid with a fruity odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 345.66 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.41 g cm −3 |
||||||||||||||||||
| Melting point |
87 ° C |
||||||||||||||||||
| solubility |
practically insoluble in water (0.1 mg l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 10 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Methoxychlor is a mixture of several structural isomeric chemical compounds from the group of chlorinated diphenylmethane derivatives . It is u. a. the p , p ′ -dimethoxy analog of p , p ′ - DDT .
Extraction and presentation
Methoxychlor can be obtained by reacting anisole with chloral in the presence of aluminum chloride or sulfuric acid.
properties
Methoxychlor is a flammable, colorless to yellowish solid with a fruity odor that is practically insoluble in water. In its pure form it is a colorless powder. The technical product is gray to yellowish and consists of 88–90% of the p , p ′ isomer. The rest consists mainly of the o , p 'isomer and up to 50 other impurities.
The compound was first described in 1944 and introduced in 1945.
toxicity
In some in vivo studies, “ technical ” methoxychlor appears to be three to four times more effective in terms of reproductive and developmental toxicity than pure methoxychlor. This is attributed to the fact that impurities in the “technical” methoxychlor, which are partly metabolites of methoxychlor, are directly estrogenic substances. Methoxychlor itself has a weak estrogenic effect.
The metabolite of methoxychlor 2,2-bis ( p -hydroxyphenyl) -1,1,1-trichloroethane (HPTE or hydroxychlor), demethylated by cytochrome P450 in the liver , turned out to be a potent endocrine disruptor . Recent studies in rats show that methoxychlor can cause diseases up to the third following generation.
use
Methoxychlor has been used as an insecticide .
Admission
In 2002 the compound was not included in the list of plant protection active ingredients permitted in the European Union . No plant protection products containing this active ingredient are permitted in the EU or Switzerland .
The approval for use as a plant protection product or biocide in the USA was withdrawn in 2003. The reason for the ban was its persistence in the environment and its ability to accumulate in the food chain .
Individual evidence
- ↑ a b c d e f g h Entry on methoxychlor in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Methoxychlor data sheet from Sigma-Aldrich , accessed on February 3, 2018 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 72-43-5 or methoxychlor ), accessed on November 2, 2015.
- ^ Entry on methoxychlor in the Hazardous Substances Data Bank , accessed July 29, 2012.
- ↑ ATSDR: Toxicological Profile for Methoxychlor , Chapter 4 .
- ↑ Robert Irving Krieger: Handbook of pesticide toxicology . tape 1 . Academic Press, 2010, ISBN 978-0-08-092201-0 ( limited preview in Google Book Search).
- Jump up ↑ Y. Hu, D. Kupfer: Metabolism of the endocrine disruptor pesticide-methoxychlor by human P450s: pathways involving a novel catechol metabolite. In: Drug metabolism and disposition: the biological fate of chemicals. Volume 30, Number 9, September 2002, pp. 1035-1042, PMID 12167570 .
- ^ The MAK Collection for Occupational Health and Safety - Methoxychlor . ISBN 978-3-527-60041-0 , doi : 10.1002 / 3527600418.mb7243d0056 .
- ↑ ATSDR: Toxicological Profile for Methoxychlor , Chapter 6 .
- ↑ scinexx.de: Pesticide still makes the great-grandchildren sick: Methoxychlor acts across generations through pathogenic changes in the genome , accessed on January 10, 2017
- ↑ Mohan Manikkam, M. Muksitul Haque, Carlos Guerrero-Bosagna, Eric E. Nilsson, Michael K. Skinner, W. Steven Ward: Pesticide Methoxychlor Promotes the Epigenetic Transgenerational Inheritance of Adult-Onset Disease through the Female Germline. In: PLoS ONE. 9, 2014, p. E102091, doi : 10.1371 / journal.pone.0102091 .
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on methoxychlor in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.
- ↑ Environmental Protection Agency : Methoxychlor Reregistration Eligibility Decision (RED) EPA Publication No. EPA 738-R-04-010 . June 30, 2004. Retrieved October 2, 2009.