Amount of substance
| Physical size | |||||||
|---|---|---|---|---|---|---|---|
| Surname | Amount of substance | ||||||
| Formula symbol | |||||||
|
|||||||
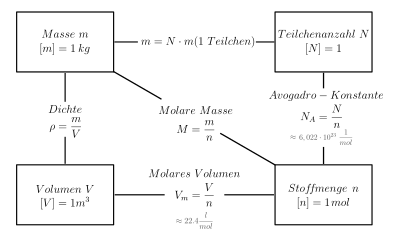
The amount of substance (outdated molar amount or molar number ) with the formula symbol is a basic quantity in the International System of Units (SI) and indirectly indicates the number of particles in a substance portion. Particles can be atoms , ions , molecules , formula units or electrons . Formula symbol and particle type X are given together as n X or n (X).
The unit of measurement for the amount of substance is the mole , an SI base unit. An amount of substance of 1 mol ( = 1 mol) contains that determined by the Avogadro constant ( N A ≈ 6 .022e23 mol −1 ) specified number of particles. The Avogadro constant is theproportionality factorbetween the amount of substance and the number of particles N(X):
Amount of substance and the quantities derived from it, such as concentration of substance , amount of substance and ratio of substance, are important in stoichiometry . The use of the quantity of substance shifts considerations of chemical reactions from the atomic or molecular range to weighable substance masses with a very high number of particles.
For the amount of substance n X and the mass m X of a substance portion of a substance X and its molar mass M X :
Calculating the amount of substance
The amount of substance is calculated
... from the crowd
The calculation from the mass is possible using the equation given above.
Example: The molar mass of water is 18 grams per mole. 9 grams of water correspond to an amount of substance of 0.5 mol.
... from the volume
One mole of an ideal gas takes up an approximate volume of V m = 22.4 l / mol under standard conditions . This volume is called the standard molar volume (molar volume). The amount of substance results directly from the volume.
Example: How many moles is equivalent to 5 liters of oxygen ?
... from the number of particles
Since the number of particles N is proportional to the amount of substance n (the proportionality factor is the Avogadro constant N A = 6.022 · 10 23 / mol), the amount of substance can be calculated from the number of particles.
Example: Given are N = 10 25 particles.
... out of concentration
Since the concentration c X (mol / l) is a measure of the concentration for solutions , which relates the amount of substance X to the volume V of the solution, this can also be calculated back to the amount of substance.
Example: How many moles of sodium chloride are in 0.22 liters of a 0.6 molar NaCl solution?
See also
Web links
Individual evidence
- ↑ Entry on amount of substance . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00297 Version: 2.3.2.