N , N , N ', N ' -tetramethylformamidinium chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | N , N , N ', N ' -tetramethylformamidinium chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 13 ClN 2 | |||||||||||||||
| Brief description |
Light yellow crystalline solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 136.62 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
soluble in water, in acetonitrile and N , N -dimethylformamide |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
N , N , N ', N ' -Tetramethylformamidiniumchlorid is the simplest representative of quaternary formamidinium cations of the general formula [R 2 N-CH = NR 2 ] + with chloride as counterion , in which all the hydrogen atoms of the protonated amidine formamidine [HC (= NH 2 ) NH 2 ] + are replaced by methyl groups .
Deprotonation produces the extremely basic carbene bis (dimethylamino) carbene of the formula R 2 N-C: -NR 2 .
Occurrence and representation
N , N , N ', N ' -Tetramethylformamidinium chloride is formed in the reaction of dimethylformamide (DMF) with dimethylcarbamoyl chloride in very high yield (95%),
The conversion of DMF with thionyl chloride in a ratio of 3: 1 provides a significantly lower yield of 72%, but a more realistic yield in view of the difficult handling of the chloride salt .
properties
N , N , N ', N ' -Tetramethylformamidinium chloride is a light yellow, strongly hygroscopic solid.
For drying, the salt is dissolved in dichloromethane and solid anhydrous sodium sulfate is added to the solution . After dissolving it several times in dichloromethane / acetone and precipitating with tetrahydrofuran , a colorless solid is obtained which is stable in the absence of air and moisture.
The assumption of a mesomeric equilibrium between ionic formamidinium chloride and covalent bis (dimethylamino) chloromethane structure
a decision was made in favor of the presence of N , N , N ', N ' -tetramethylformamidinium chloride by reacting with germanium (II) chloride or tin (II) chloride .
The extreme hygroscopicity of the chloride salt complicates the handling of the compound considerably. Therefore, syntheses of the much more manageable salts N , N , N ', N ' -tetramethylformamidinium methyl sulfate from the dimethylformamide-dimethyl sulfate complex and of N , N , N ', N ' -tetramethylformamidinium p-toluenesulfonate from DMF and p -Toluenesulfonic acid chloride described.
Applications
N , N , N ', N ' -Tetramethylformamidinium chloride is a suitable reagent for aminomethylenation; H. for the introduction of a = CH-NR 1 R 2 function on CH-acidic compounds. For example, ethyl cyanoacetate reacts with the formamidinium salt in the presence of solid sodium hydroxide, practically quantitatively, to form ethyl (dimethylaminomethylene) cyanoacetate.
The aminomethylenation provides intermediates for the synthesis of heterocycles , such as. B. indoles , pyrimidines , pyridines and quinolones .
From N , N , N ', N ' -Tetramethylformamidiniumchlorid is formed with alkali metal dimethylamides, such as. B. lithium dimethylamide or sodium dimethylamide tris (dimethylamino) methane in yields of 55-84%.
The reaction product is also useful as a reagent for formylation and aminomethylenation.
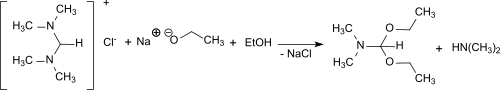
From N , N , N ', N ' tetramethylformamidinium chloride and sodium ethylate in ethanol , dimethylformamide diethylacetal is formed in 68% yield.
The N , N , N ', N ' -tetramethylformamidinium salt reacts with aqueous sodium cyanide to form bis (dimethylamino) acetonitrile>
With anhydrous hydrocyanic acid , N , N , N ', N ' -tetramethylformamidinium chloride is converted into dimethylaminomalonic acid dinitrile in 92% yield
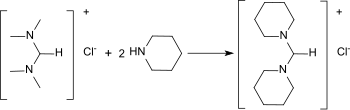
N , N , N ', N ' -Tetramethylformamidinium chloride can be re-gaminated with cycloaliphatic amines to give the corresponding heterocyclic formamidines.
More recently, the use of N , N , N ', N ' -tetramethylformamidinium chloride as a catalyst in the preparation of carboxylic acid chlorides from carboxylic acids and phosgene has been reported.
Strong bases such as B. Phenyllithium can abstract a proton from the formamidinium cation of N , N , N ', N ' -Tetramethylformamidiniumchlorid to form bis (dimethylamino) carbene.
Individual evidence
- ↑ a b c d data sheet N, N, N ′, N′-tetramethylformamidinium chloride from Sigma-Aldrich , accessed on October 20, 2016 ( PDF ).
- ↑ a b c W. Kantlehner, P. Speh: acid amide reactions. LI. Note on the preparation of N, N, N ′, N′-tetramethylformamidinium chloride . In: Chem. Ber. tape 104 , no. 11 , 1971, p. 3714-3715 , doi : 10.1002 / cber.19711041136 .
- ^ A b c Z. Arnold: The preparation of tetramethylformamidinium salts and their vinylogues . In: Collect. Czech. Chem. Commun. tape 24 , 1959, pp. 760-765 , doi : 10.1135 / cccc19590760 .
- ↑ a b c d R.W. Alder, ME Blake, S. Bufali, CP Butts, AG Orpen, J. Schütz, SJ Williams: Preparation of tetraalkylformamidinium salts and related species as precursors to stable carbenes . In: J. Chem. Soc., Perkin Trans. Volume 1 , 2001, p. 1586–1593 , doi : 10.1039 / B104110J .
- ↑ a b A.M. Magill, KJ Cavell, BF Yates: Basicity of nucleophilic carbenes in aqueous and nonaqueous solvents - theoretical predictions . In: J. Am. Chem. Soc. tape 126 , no. 28 , 2004, pp. 8717-8724 , doi : 10.1021 / ja038973x .
- ↑ a b Patent DE1205528 : Process for the production of N-substituted amidines or their vinylogues. Registered on February 8, 1962 , published on November 25, 1965 , applicant: H. Bredereck, inventor: H. Bredereck, F. Effenberger, G. Simchen.
- ↑ X. Tian, T. Pape, NW Mitzel: Formamidinium Salts of Low Valent Metal Halide Anions MX 3 - (M = Ge, Sn) and M 2 X 6 2- (M = Ga, In) . In: Z. Naturforsch. 59b, no. 11-12 , 2004, pp. 1524-1531 , doi : 10.1515 / znb-2004-11-1224 .
- ↑ H. Bredereck, F. Effenberger, G. Simchen: acid amide reactions, XXXII. About acid amide dialkyl sulfate complexes . In: Chem. Ber. tape 96 , no. 5 , 1963, pp. 1350-1355 , doi : 10.1002 / cber.19630960526 .
- ↑ Patent US3707553 : Tetramethylformamidinium arenesulfonates and method of preparation. Filed August 24, 1965 , published December 26, 1972 , Applicant: Armstrong Cork Co., Inventor: GE Bagley, AC Poshkus.
- ↑ H. Schindlbauer: Reactions with dimethylformamide, 3rd edition. The reaction of arylsulfochlorides and arylsulfonic acids with dimethylformamide . In: monthly Chem. Band 100 , no. 5 , 1969, p. 1590-1595 , doi : 10.1007 / BF00900174 .
- ↑ Patent US5241099 : Process for the preparation of aminomethylene compounds. Registered on March 16, 1992 , published on August 31, 1993 , applicant: Bayer AG, inventor: H.-U. Blank, H. Kraus.
- ↑ H. Bredereck, F. Effenberger, T. Brendle: Synthesis and reactions of Trisdimethylaminomethan . In: Angew. Chem. Band 78 , no. 2 , 1966, p. 147-148 , doi : 10.1002 / anie.19660780212 .
- ↑ Patent DE1217391 : Process for the production of tris-dimethylaminomethane. Applied on September 29, 1964 , published on December 8, 1966 , applicant: H. Bredereck, inventor: H. Bredereck, F. Effenberger, T. Brendle.
- ↑ H. Bredereck, F. Effenberger, T. Brendle, H. Muffler: Orthoamide, V. Synthesis of Tris-dialkylamino-methanes . In: Chem. Ber. tape 101 , no. 5 , 1968, p. 1885–1888 , doi : 10.1002 / cber.19681010541 .
- ↑ H. Gold: The reaction of cyanuric chloride with dimethylformamide . In: Angew. Chem. Band 72 , no. 24 , 1960, pp. 956-959 , doi : 10.1002 / anie.19600722406 .
- ↑ H. Bredereck, G. Simchen, W. Kantlehner: Orthoamide, XVI. Synthesis of ON- and NN-acetals of the α-keto-carboxylic acid nitriles and of imino esters . In: Chem. Ber. tape 104 , no. 3 , 1971, p. 924-931 , doi : 10.1002 / cber.19711040331 .
- ^ A b H. Gold, O. Bayer: The representation of basic substituted malonic acid dinitrile . In: Chem. Ber. tape 94 , no. 10 , 1961, pp. 2594-2596 , doi : 10.1002 / cber.19610941004 .
- ↑ Patent EP1124783A1 : Method for producing carboxylic acid chlorides. Applied on November 4, 1998 , published on August 22, 2001 , applicant: BASF AG, inventor: J. Henkelmann, A. Stamm.