N- phenyl-2-naphthylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
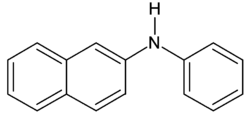
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | N- phenyl-2-naphthylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 13 N | ||||||||||||||||||
| Brief description |
almost colorless and odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 219.29 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.24 g cm −3 |
||||||||||||||||||
| Melting point |
108 ° C |
||||||||||||||||||
| boiling point |
400 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
N- phenyl-2-naphthylamine is a chemical compound from the aminobenzenes group .
Extraction and presentation
N can phenyl-2-naphthylamine by reaction of 2-naphthol with aniline - hydrochloride are obtained, which already in 1880 by Carl Gräbe was published.
properties
N- phenyl-2-naphthylamine is a flammable, hardly inflammable, almost colorless, odorless, flaky solid that is practically insoluble in water and turns gray-pink in air.
use
N- phenyl-2-naphthylamine is used as an antioxidant ( e.g. for Semtex ) and stabilizer (for 1,3-butadiene ).
Individual evidence
- ↑ a b c d e f g h Entry on N-phenyl-2-naphthylamine in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ International Agency for Cancer Research (Ed.): Some Aromatic Amines and Related Nitro Compounds - Hair Dyes, Coloring Agents & Miscellaneous Industrial Chemicals (= IARC Monographs . Volume 16 ). 1978, p. 326 ( PDF - manufacturer information BFGoodrich ).
- ↑ Entry on N-2-naphthylaniline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 2, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet N-Phenyl-2-naphthylamine, 97% from Sigma-Aldrich , accessed on December 31, 2016 ( PDF ).
- ↑ C. Graebe: About the reactivity of naphthols . In: Reports of the German Chemical Society . tape 13 , no. 2 , 1880, p. 1849–1851 , doi : 10.1002 / cber.188001302155 : “About two years ago, Mr. R. Holdmann made the peculiar observation in Caro's laboratory that β-naphtol, when heated with hydrochloric aniline, yields a new nitrogen-containing body. "
- ↑ Patent DE14612 : Process for converting the naphthols into their corresponding primary, secondary and tertiary monamines. Registered on February 22, 1880 , published on July 15, 1881 , applicant: Badische Anilin- und Sodafabrik.
- ^ Paul M. Pellegrino, Ellen L. Holthoff, Mikella E. Farrell: Laser-Based Optical Detection of Explosives . CRC Press, 2015, ISBN 978-1-4822-3329-2 , pp. 76 ( limited preview in Google Book search).
- ↑ Entry on N-phenyl-2-naphthylamine in the Hazardous Substances Data Bank , accessed on December 31, 2016.


