N -phenylglycine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
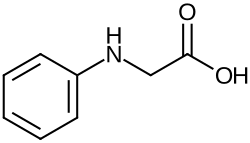
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | N-phenylglycine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 9 NO 2 | ||||||||||||||||||
| Brief description |
colorless to beige odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 151.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.145 g cm −3 (19.5 ° C) |
||||||||||||||||||
| Melting point |
127-128 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
N- phenylglycine is a chemical compound from the group of aminobenzenes and aminocarboxylic acids .
Extraction and presentation
N- phenylglycine can be obtained by reacting aniline and chloroacetic acid . It can also be produced from aniline, formaldehyde , sodium hydrogen sulfite and hydrocyanic acid with subsequent saponification of the nitrile anilinoacetonitrile .
properties
N- phenylglycine is a colorless to beige odorless solid that is sparingly soluble in water. It gives water-soluble salts with alkali hydroxides. It is a selective antagonist of metabotropic glutamate receptors .
use
N- phenylglycine is an intermediate product in the synthesis of indigo according to Heumann's indigo synthesis . It is also used in the synthesis of pharmaceuticals, is used to produce photosensitive layers on lithographic printing plates and is a component in liquid-crystalline films. The compound is also used in tooth filling materials and adhesives.
Individual evidence
- ↑ a b c d e f g Entry on N-phenylglycine in the GESTIS substance database of the IFA , accessed on January 15, 2016(JavaScript required) .
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons . William Andrew, 2014, ISBN 978-0-323-29060-9 , pp. 295 ( limited preview in Google Book search).
- ↑ a b c d e Entry on Phenylglycine. In: Römpp Online . Georg Thieme Verlag, accessed on January 15, 2016.
- ↑ a b N-Phenylglycine - Lexicon of Chemistry . Spektrum.de, accessed on January 15, 2016.