Norovirus
| Norovirus | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

3D model of a single norovirus virion |
||||||||||||||||||
| Systematics | ||||||||||||||||||
|
||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||
|
||||||||||||||||||
| Scientific name | ||||||||||||||||||
| Norovirus | ||||||||||||||||||
| Left | ||||||||||||||||||
|
The genus Norovirus comprises non-enveloped viruses with a single-stranded RNA with positive polarity (ss (+) RNA) from the family Caliciviridae . To date, various norovirus species have been discovered in humans as well as in cattle , pigs , mice and oysters . The human noroviruses in particular are of great medical importance as pathogens for viral gastroenteritis .
The name Norovirus is derived from Norwalk virus , the name for the type species of the genus.
Initial description
The type species of the genus Norovirus , the Norwalk virus , was first morphologically characterized in stool samples from a viral gastroenteritis outbreak from 1968 in Norwalk , Ohio , by immunoelectron microscopy in 1972 . In order to be able to prove the connection between the virus found and a disease of gastroenteritis, purified stool ultrafiltrate (obtained from human feces of sick patients) was administered orally to volunteers who then also got sick.
morphology
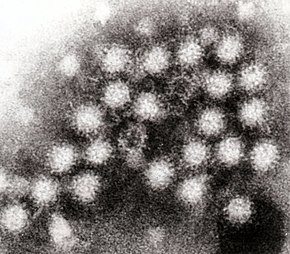
Noroviruses consist of an unenveloped, icosahedral (twenty surface) capsid , which is formed by the main capsid protein VP1. VP1 proteins can be assembled to form larger capsids that consist of 180 proteins, have a diameter of 38 to 40 nm and have T = 3 symmetry ; they can also assemble into smaller capsids (23 nm) from 60 VP1 proteins with T = 1 symmetry. Both structures are shown in the electron microscope image as round structures with a blurred edge (see illustration).
A secondary capsid protein , which is located on the inside, is also involved in the construction of the protein capsule of a virion . Biochemical studies showed that it binds to the inner side of the capsid and increases the stability of the capsid. Inside the capsid, the virus genome is present as positive single-stranded RNA (ss (+) RNA). The VPg protein is covalently bound to the 5 'end of the RNA of the genome, its 3' end is polyadenylated .
Genome
The single-stranded RNA genome of the noroviruses is about 7.3 to 7.7 kb in size and comprises three partially overlapping open reading frames (ORFs).
The ORF1 codes for a polyprotein which, after translation , is cut into six non-structural proteins by a viral protease (NS 1/2 - NS 7; the numbering corresponds to the arrangement of the genes coding for the proteins on the genome). These include viral RNA polymerase (NS 7) and viral protease (NS 6). The capsid protein VP1 is encoded by ORF2 and the virion-associated structural protein VP2 of unknown function is encoded by ORF3. When a cell is infected with different strains or variants, the genome of the noroviruses can very effectively produce new variants and subtypes through recombination . A the influenza viruses of similar antigenic drift and antigenic shift are observed in some species of noroviruses. A hypervariable region is located in the P2 domain of the VP1 protein, which is on the outside of the virus capsid and serves as a binding site for antibodies, and is therefore exposed to particularly strong selection by the immune system.
In a 2017 study, Parra and Green's group sifted through extensive data sets on human norovirus infections and compared the ss (+) RNA of over 20 genotypes of both genotype groups I and II with regard to the mutations that had accumulated over the years. While the genome of the other genotypes examined remained relatively stable, the norovirus of type GII.4 changed rapidly. It evolved both in one host and alternately from one host to another; Numerous variants of this genotype with different VP1 protein were created in particular by changing the sequence in ORF2. In contrast, the other genotypes, which are more static, can be grouped into several subgroups with regard to their antigens, which may correspond to an “immunotype”, which would simplify the development of vaccines.
Replication cycle
The replication cycle is only partially understood. Most of the experiments were performed with the murine norovirus subtype. It is assumed that histo blood group antigens, to which the AB0 system of blood groups, the precursor substance H and the Lewis antigens belong, are crucial for the binding of the virus to its host cell. The antigens are present on red blood cells as well as on epithelial and endothelial cells. Cell tropism has been observed for Murine Norovirus, the virus infects both lymphocytes and enterocytes. Human norovirus subtypes could be replicated in vitro both in B-lymphocytes and in intestinal organoids grown from stem cells , but only in small amounts.
After the virus has been taken up by the host cell, the viral mRNA is read and translated by the host's own RNA polymerase. VPg (NS 5), which is covalently bound to the 5 'end of the mRNA strand, serves as a protein primer. In addition, VPg plays a role in the initiation of translation. Translation of the individual proteins occurs through leaky scanning and translation re-initiation. The viral protease (NS 6) divides the new polyprotein into individual non-structural proteins that are required for the virus to replicate. Viral mRNA is duplicated in the cytoplasm by the RNA-dependent RNA polymerase (NS 7). Since this, like other RNA viruses, has no corrective function, the newly generated RNA has a significantly increased mutation rate. (Incorporation of one mutation per 10,000 bp). This leads to the formation of quasi-species which allow rapid adaptation and immune evasion. The capsid protein VP1 forms the capsid in which the viral mRNA is packaged before the virus leaves the cell.
Immunoregulatory functions have been observed for the non-structural proteins NS1 / 2, NS 3 and NS4, also known under the names p48, NTPase and p22.
Systematics
- Genus Norovirus (obsolete Norwalk-like virus )
- Species Norwalk virus ( Humanes Norovirus , en. Norwalk virus )
- Candidates classified provisionally (by proposal):
- Species ' human norovirus alphatron '
- Species ' human norovirus Saitama '
- Species ' Bovine Norovirus-CH126'
- Species ' Bovine Norovirus-Jena '
- Species ' Murine Norovirus 1 '
- Species ' Porcine Norovirus ' (swine norovirus)
- Species ' norovirus of the oyster '
Human noroviruses
For information about the clinical course, transmission, epidemiology etc. see human noroviruses ( Norwalk virus ).
literature
- MK Koopmans et al .: Genus Norovirus . In: CM Fauquet, MA Mayo et al .: Virus taxonomy: classification and nomenclature of viruses: eighth report of the International Committee on the Taxonomy of Viruses. Academic Press, London / San Diego 2005, pp. 847f.
Web links
Individual evidence
- ↑ ICTV Master Species List 2018b v1 MSL # 34, Feb. 2019
- ↑ a b c d e ICTV: ICTV Taxonomy history: Rabbit hemorrhagic disease virus , EC 51, Berlin, Germany, July 2019; Email ratification March 2020 (MSL # 35)
- ↑ Juliana D. Siqueira, Maria Gloria Dominguez-Bello, Monica Contreras, Orlana Lander, Eric Delwart: Complex virome in feces from Amerindian children in isolated Amazonian villages. In: Nature Communications 9 (1), December 2018, doi: 10.1038 / s41467-018-06502-9
- ↑ Aase B Mikalsen, Pål Nilsen, Marianne Frøystad-Saugen, Karine Lindmo, Trygve M Eliassen, Marit Rode, Øystein Evensen: Characterization of a Novel Calicivirus Causing Systemic Infection in Atlantic Salmon (Salmo salar L.): Proposal for a New Genus of Caliciviridae. In: PLoS ONE 9 (9): e107132, September 2014, doi: 10.1371 / journal.pone.0107132
- ^ R. Dolin, NR Blacklow et al .: Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proceedings of the Society of Experimental Biological Medicine '(1972) Volume 140, Number 2, pp. 578-583 PMID 4624851 .
- ↑ a b c Caliciviridae - ICTV Ninth Report .
- ↑ White LJ, Hardy ME, Estes MK: Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. Journal of Virology, October 1997, PMC 192173 (free full text).
- ↑ Lewis DC: Three serotypes of norwalk ‐ like virus demonstrated by solid ‐ phase immune electron microscopy. Journal of Medical Virology, January 1990, doi : 10.1002 / jmv.1890300117 .
- ↑ Sompong Vongpunsawad, BV Venkataram Prasad, Mary K. Estes: Norwalk virus capsid protein VP2 Minor Associates within the VP1 domain shell. Journal of Virology, April 2013, doi : 10.1128 / JVI.03508-12 .
- ↑ Lin Y, Fengling L, Lianzhu W, Yuxiu Z, Yanhua J: Function of VP2 protein in the stability of the secondary structure of virus-like particles of genogroup II norovirus at different pH levels: function of VP2 protein in the stability of NoV VLPs. Journal of Microbiology, October 3, 2014, doi : 10.1007 / s12275-014-4323-6 .
- ↑ Allen DJ, Gray JJ, Gallimore CI, Xerry J, Iturriza-Gómara M: Analysis of Amino Acid Variation in the P2 Domain of the GII-4 Norovirus VP1 Protein Reveals Putative Variant-Specific Epitopes. PLOS ONE, January 23, 2008, doi : 10.1371 / journal.pone.0001485 .
- ↑ Gabriel I. Parra, R. Burke Squires, Consolee K. Karangwa, Jordan A. Johnson, Cara J. Lepore, Stanislav V. Sosnovtsev, Kim Y. Green: Static and evolving Norovirus genotypes: Implications for epidemiology and immunity. In: PLoS Pathogens 2017/13 (1) e1006136. doi: 10.1371 / journal.ppat.1006136 (free full text).
- ^ Graziano VR, Wei J, Wilen CB: Norovirus Attachment and Entry . In: Noroviruses . Viruses, May 30, 2019, doi : 10.3390 / v11060495 .
- ↑ Julie E Heggelund, Annabelle Varrot, Anne Imberty, Ute Krengel: Histo-blood group antigens as mediators of infections. Current Opinion in Structural Biology, June 2017, doi : 10.1016 / j.sbi.2017.04.001 .
- ^ S. Marionneau, A. Cailleau-Thomas, J. Rocher, B. Le Moullac-Vaidye, N. Ruvoën, M. Clément, J. Le Pendu: ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochemistry, July 2001, doi : 10.1016 / S0300-9084 (01) 01321-9 .
- ^ Christiane E. Wobus: The Dual Tropism of Noroviruses . Journal of Virology, July 31, 2018, doi : 10.1128 / JVI.01010-17 .
- ↑ Costantini V, Morantz EK, Browne H, et al .: Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation . Emerging Infectious Diseases, August 2018, doi : 10.3201 / eid2408.180126 .
- ↑ Melissa K Jones, Katrina R Grau, Veronica Costantini, et al .: Human norovirus culture in B cells . Nature Protocols, June 1, 2016, doi : 10.1038 / nprot.2015.121 .
- ↑ a b c d Lucy G. Thorne, Ian G. Goodfellow: Norovirus gene expression and replication. Journal of General Virology, February 1, 2014, doi : 10.1099 / vir.0.059634-0 .
- ↑ a b Michele E. Hardy: Norovirus protein structure and function. FEMS Microbiology Letters, December 1, 2005, doi : 10.1016 / j.femsle.2005.08.031 .
- ↑ Eric F. Donaldson, Lisa C. Lindesmith, Anna D. LoBue & Ralph S. Baric: Viral shape-shifting: norovirus evasion of the human immune system. Nature Reviews Microbiology, February 2, 2010, doi : 10.1038 / nrmicro2296 .