Phenoxyacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Phenoxyacetic acid | ||||||||||||||||||
| other names |
2-phenoxyacetic acid ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 8 H 8 O 3 | ||||||||||||||||||
| Brief description |
white to beige powder, needle-shaped crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
98-100 ° C |
||||||||||||||||||
| boiling point |
285 ° C |
||||||||||||||||||
| pK s value |
3.17 (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Phenoxyacetic acid is an organic compound that smells like honey. It forms colorless, needle-shaped crystals that are difficult to dissolve in water and easily soluble in glacial acetic acid, alcohol and ether. The substance is a medium strong acid (pK s = 3.17) and has a strong antiseptic.
presentation
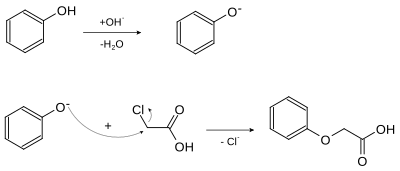
Phenoxyacetic acid can be obtained from the reaction of phenol and chloroacetic acid with sodium hydroxide solution . The sodium hydroxide solution deprotonates the hydroxyl group of the phenol. The resulting phenolate now attacks nucleophilically on the β - carbon atom of chloroacetic acid, splitting off a chloride ion .
Individual evidence
- ↑ a b c d e f g Data sheet phenoxyacetic acid (PDF) from Merck , accessed on December 26, 2019.
- ↑ a b Wissenschaft-Online-Lexika: Entry on phenoxyacetic acid in the Lexikon der Chemie , accessed September 6, 2008.
- ^ Entry on phenoxyacetic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Gigiena i Sanitariya. (HYSAAV) Vol. 46 (1), 1981, p. 25.
- ^ Food and Cosmetics Toxicology . Vol. 17, 1979, p. 887.
- ↑ P. Giacosa: Advantageous presentation of phenolglycolic acid and pyrogallol triglycolic acid , in: J. Prakt. Chem. , 1879 , 19 , pp. 396-399.