Polyphosphazenes
As polyphosphazenes a class of hybrid is inorganic - organic polymers designated with backbones consisting of alternating phosphorus - and nitrogen atoms formed with alternating single and double bonds and formal (NPR with the formula 1 R 2 ) n will be described. The phosphorus atoms carry two identical (R 1 = R 2 ) or different (R 1 ≠ R 2 ) substituents, such as. B. alkoxy , amino , dialkyl or diarylamino (R 2 N-) or halogen radicals (such as chlorine or fluorine atoms ), which have a significant influence on the extraordinary properties of polyphosphazenes.
Polyphosphazenes are generally linear polymers, the standard by ring-opening polymerization of ring-opening polymerization ROP of the cyclic Hexachlorphosphazens and subsequent nucleophilic substitution of the chlorine atoms produced. There are also so-called cyclolineare , i.e. H. Phosphazene rings linked by linear segments (mostly soluble and flexible) and two-dimensionally linked, so-called cyclomatrix phosphazenes (insoluble and rigid).
As with organic polymers, block copolymers , comb polymers and dendrimers are also known from polyphosphazenes .
Fundamental work on polyphosphazenes has come from Harry R. Allcock's group at Pennsylvania State University, State College, PA, from 1965 to the present day.
synthesis
Polyorganophosphazenes (NPR 1 R 2 ) n from polydichlorophosphazenes (NPCl 2 ) n
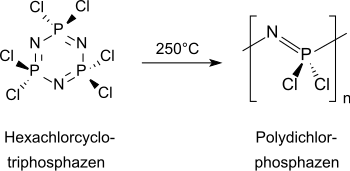
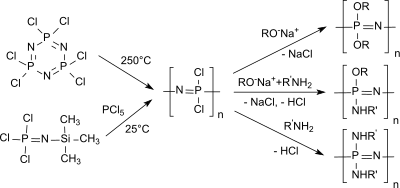
The standard method for the synthesis of polyorganophosphazenes is a two-step process, starting from the six-membered cyclic inorganic hexachlorocyclotriphosphazene (NPCl 2 ) 3 , which is present in sufficient purity as a crystalline solid after recrystallization and sublimation .
In the first step, when heated to over 250 ° C in a closed system, the low molecular weight cyclophosphazene is converted into high molecular weight (Mw 10 5 - 10 6 and higher) linear polydichlorophosphazene and, if the conversion is below 70%, uncrosslinked. The linear polydichlorophosphazenes obtained in relatively low yields are soluble in benzene , toluene and tetrahydrofuran , but are unstable even in moist air and crosslink to form insoluble products. The undesired crosslinking of polydichlorophosphazenes is effectively prevented by dissolving them in diglyme .
However, the drastic polymerization conditions do not allow any control of the molar masses and the molar mass distribution (polydispersity).
The polycondensation of trichlor [(dichlorophosphoryl) imido] phosphorane Cl 3 P = NP (O) Cl 2 , when heated for several hours at 280 ° C. in the presence of PCl 5, yields linear polydichlorophosphazene with a broad molecular weight distribution. The required reaction conditions make the synthesis unusable for an industrial process.
The reaction of phosphorus trichloride , chlorine and ammonia in chlorobenzene at 130 ° C. produces high yields of linear liquid oligodichlorophosphazenes with a degree of polymerization of up to 9 and broad polydispersity, which have not found any technical interest.
Another alternative is the fluorine-analogous polydifluorophosphazene (NPF 2 ) n , which, however, requires polymerization temperatures of 350 ° C and is insoluble in common solvents, but is particularly suitable for the reaction with organometallic compounds to form polyalkyl or polyarylphosphazenes with PC bonds .
Polyphosphazenes by the cationic polymerization of phosphoranimines
Linear polydichlorophosphazene with defined molecular weights and narrow molecular weight distribution is accessible by polymerization of trichlorophosphoranimines, such as. B. Cl 3 P = N-SiMe 3 ,
which can also be carried out as a quasi-living cationic polymerization in the presence of traces of phosphorus pentachloride PCl 5 .
In this way, more complicated polymer architectures such as block copolymers , comb polymers, star polymers or dendrimers can be realized.
Polyphosphazenes by anionic polymerization of phosphoranimines
Phosphoranimines of the type Me 3 SiN = P (OCH 2 CF 3 ) (OR) 2 can also under less drastic conditions (below 100 ° C) anionic by fluoride ions with the initiator ( tetrabutylammonium fluoride ) Bu 4 N + F - to polyphosphazenes with relatively close Polydispersity (<1.4) and adjustable molar masses from 10,000 to 200,000 can be anionically polymerized .
Even more electronegative alkoxyphosphazenes, such as. B. Tris (2,2-dinitropropoxy) -N- (trimethylsilyl) phosphoranimine, polymerize with Bu 4 N + F - to polyphosphazenes even at room temperature . However, the synthesis of the starting compounds is associated with considerable preparative effort.
Synthesis of polyorganophosphazenes
In the second stage of the standard procedure using polydichlorophosphazenes, the chlorine atoms on (PNCl 2 ) n are replaced by organic radicals (alkoxy, aryloxy, amino) or metal organyls in a nucleophilic substitution .
By combining several nucleophilic reactants, a large number of polyorganophosphazenes with very different properties can be generated.
Polyorganophosphazenes (NPR 1 R 2 ) n by polycondensation of reactive PN monomers
Instead of ring-opening polymerization of cyclic (NPCl 2 ) 3 and subsequent (complete) substitution of the labile chlorine atoms, (stable) linear polyorganophosphazenes are also made directly from phosphoranimines bearing organic residues
or phosphine azides R 2 PN 3 accessible.
In most cases, controlled high molar masses and narrow polydispersities cannot be produced even on these synthesis routes, which require parameters important for technical applications such as high strength with good processability and defined phase transitions.
The route via the relatively easily accessible trichloro (trimethylsilyl) phosphoraneimine Cl 3 P = N-SiMe 3 (from lithium bis (trimethylsilyl) amide and PCl 5 ) seems to offer a useful compromise . In addition, the alternative syntheses often require expensive and dangerous (e.g. azide ) reagents and considerable preparative effort, which stands in the way of a commercial breakthrough for the defined polyphosphazenes described in this way.
The polyphosphazene class of substances has met with great interest in academic research because of its extraordinary properties, so that by 2004 more than 700 different polyphosphazenes had been presented and described.
properties
The different polyphosphazenes owe their high flexibility, extraordinary thermal stability and fire resistance, high refractive index , resistance to high- energy radiation, as well as permeability to UV and visible light to the polymer backbone, which is highly flexible due to the bond distances and angles - [- N = P (R 1 R 2 ) -] n -. The variation of the side groups on the polyphosphazene chain determines the hydrophilicity or hydrophobicity , the chemical stability, the permeability for solvents, the mechanical strength and elasticity, the broad range of the glass transition temperature T G between −100 ° C and +180 ° C and the Biocompatibility of polyphosphazenes.
Applications
Elastomers
Fluoroalkoxy-substituted elastomeric polyphosphazenes of the type PN-F with two side groups of different lengths are amorphous and, because of their resistance to fuels , oils and hydraulic fluids, were used in the temperature range between −60 ° C and +175 ° C under the brand name Eypel R F as the base material for O-rings and seals in aerospace applications.
The arylalkoxy polyphosphazene PN-A was offered as a cable insulation material because of its fire retardant properties. The commercialization of the Eypel types failed because of their exorbitant prices even for military applications; production was therefore discontinued by the Ethyl Corporation in the 1990s .
Two different flexible page groups, such as B. alkoxy, aryloxy, or oligo-ethyleneoxy groups in an arbitrary sequence along the -N = PN = P chain prevent crystallization and allow the flexible mobility of the polymer backbone and thus also lead to elastomers. The variation of the side groups produces polyorganophosphazenes with hydrophobic (alkyloxy) to hydrophilic water-soluble (oligoethyleneoxy) properties with glass transition temperatures between −100 ° C and +100 ° C.
Thermoplastics
Polyorganophosphazene with two identical side groups, such as. B. Methoxy (CH 3 O), ethoxy (CH 3 CH 2 O), phenoxy (C 6 H 5 O), amino (H 2 N, RHN, R 2 N) as well as the early “workhorse” of the Allcock group trifluoroethoxy (CF 3 CH 2 O) are usually thermoplastic materials. In particular, the poly [bis (2,2,2-trifluoroethoxy) phosphazene] has been intensively investigated because of its pronounced hydrophobicity, biocompatibility, as well as fire resistance and radiation stability and because of its suitability for the production of microfibers , films and membranes .
Polymer electrolytes
Linear polyphosphazenes with pendant oligoethyleneoxy groups, e.g. B. MEEP, have been investigated for many years for their possible uses as solid (and refractory) polymer electrolytes in rechargeable lithium-ion batteries , as they are good solvents for conductive salts, such as. B. lithium trifluoromethane sulfonate (lithium triflate, Li + CF 3 SO 3 - ), lithium bis (trifluoromethanesulfonate) imide (LiTFSI, Li + (CF 3 SO 3 - ) 2 N) or lithium bis (oxalato) borate (LiBOB, Li + B (C 2 O 4 ) 2 - ) and have useful ionic conductivities.
Networked Polyaryloxyphosphazene with sulfonic acid - or phosphonic because of their proton conductivity as membranes for proton exchange membrane fuel cells (English proton exchange membrane fuel cell, PEMFC.) And because of their low methanol permeability for direct methanol fuel cells (DMFC) of interest.
Membranes and hydrogels
Polyorganophosphazenes have also been considered as materials for the production of gas separation membranes and pervaporation membranes.
Water-soluble polyphosphazenes with oligoethyleneoxy side groups can be crosslinked by irradiation with gamma rays . The crosslinked polyorganophosphazenes swell with water to form hydrogels , which undergo a sol-gel phase transition when the temperature changes , i. H. expand below a critical temperature and contract above it and can be used for the controlled release of active ingredients.
The introduction of polymerizable groups, such as. B. acrylic acid ester functions , in the oligoethylene glycol side groups allows subsequent crosslinking by UV radiation and provides hydrogels with improved mechanical stability
Polyphosphazenes containing carboxylate groups can also be prepared by adding divalent cations, such as. B. calcium ions Ca 2+ are reversibly crosslinked to form hydrogels and open up further access to implants with controlled drug release.
Implant materials
The extreme variability in the chemical structure (side groups on the P atom of the polymer chain) and in the molecular architecture of possible copolymer structures of polyorganophosphazenes as well as their generally good biocompatibility enable the targeted construction of biomedical materials for short and long-term applications in the physiological environment. A large number of polyorganophosphazenes have been used as macromolecular drug carriers, as membranes for controlled drug delivery , as biostable elastomeric framework materials, in particular with amino acid side groups , and as biodegradable framework materials for building up bone substance and replacing tissue . tissue engineering ). The polyorganophosphazenes - depending on the chemical nature of the side chains and the molecular architecture - are completely degraded to ammonium and phosphate ions within weeks or months .
Despite intensive research and development work since Justus von Liebig's first observations in 1832, polyorganophosphazenes - in contrast to the similar polyorganosiloxanes - have so far not found any sustainable commercial technical applications. HR Allcock gives a current reason for this in 2014: Perhaps the greatest impediment to wider commercialization is the difference in chemistry compared to that used in conventional petrochemical polymer manufacturing . ("Perhaps the biggest barrier to widespread commercialization is the difference in chemistry compared to that used in making conventional petrochemical polymers.")
Due to the demanding syntheses for the functional monomers, the complex polymer-analogous conversions to the polymers, as well as the pronounced tendency of the intermediate stages, in particular the halogen-containing prepolymers of the type (NPCl 2 ) n , to hydrolysis and crosslinking, polyphosphazenes will probably continue to remain interesting exotic among the polymers .
literature
- HR Allcock: Chapter 7: Phosphazene High Polymers, in RSCA Polym. Chem. Ser. No 11 Phosphorus-based polymers: from synthesis to applications . Ed .: S. Monge, G. David. 2014, ISBN 978-1-84973-646-6 , pp. 125-150 .
- AK Andrianov: Polyphosphazenes for biomedical applications . Wiley, 2009, ISBN 978-0-470-19343-3 .
- JE Mark, HR Allcock, R. West: Inorganic Polymers . 2nd Edition. Oxford University Press, 2005, ISBN 0-19-513119-3 .
- V. Chandrasekhar: Inorganic and organometallic polymers . Springer, 2005, ISBN 3-540-22574-9 , doi : 10.1007 / b137079 .
- M. Gleria, R. De Jaeger, P. Potin: Synthesis and characterization of poly (organophosphazenes) . Nova Science Publishers, 2004, ISBN 1-59454-024-1 .
- M. Gleria, R. De Jaeger: Polyphosphazenes: A worldwide insight . Nova Science Publishers, 2004, ISBN 1-59454-024-1 .
- HR Allcock: Chemistry and Applications of Polyphosphazenes . Wiley-Interscience, 2003, ISBN 0-471-44371-9 .
Web links
Individual evidence
- ^ P. Potin, R. De Jaeger: Polyphosphazenes: Synthesis, structures, properties, applications . In: Eur. Polym. J. Band 27 , no. 4-5 , 1991, pp. 341-348 , doi : 10.1016 / 0014-3057 (91) 90185-Q .
- ↑ a b F.F. Stewart, TA Luther, MK Harrup, CJ Orne: Phosphazene: A Worldwide Insight, Chapter 24: Linear and Cyclomatrix Polyphosphazene Research for Membrane Applications . Ed .: M. Gleria, R. De Jaeger. Nova Science Publishers, 2004, ISBN 1-59033-423-X , pp. 573-590 .
- ↑ a b S.Y. Cho, HR Allcock: Dendrimers Derived from Polyphosphazene - Poly (propyleneimine) Systems: Encapsulation and Triggered Release of Hydrophobic Guest Molecules . In: Macromolecules . tape 40 , no. 9 , 2007, p. 3115-3121 , doi : 10.1021 / ma062582w .
- ↑ HR Allcock, RL Kugel: Synthesis of high polymeric alkoxy- and aryloxyphosphonitriles . In: J. Amer. Chem. Soc. tape 87 , no. 18 , 1965, p. 4216-4217 , doi : 10.1021 / ja01096a056 .
- ↑ RJ Davidson, EW Ainscough, AM Brodie, MR Waterland, HR Allcock, MD Hindenlang, GNL Jameson: Avoiding Crosslinking in Iron-Polyphosphazene Metallo-Polymers . In: Inorg. Chem. Commun. tape 51 , 2015, p. 1–3 , doi : 10.1016 / j.inoche.2014.10.011 .
- ↑ Harry R. Allcock. on the Pennsylvania State University website.
- ↑ R. Koci Voznicova, J. Taraba, J. Prihoda, M. Alberti: The synthesis and characterization of new aminoadamantane derivatives of hexachloro-cyclo-triphosphazene . In: Polyhedron . tape 27 , 2008, p. 2077-2082 , doi : 10.1016 / j.poly.2008.04.001 .
- ^ AK Andrianov, J. Chen, MP LeGolvan: Poly (dichlorophosphazene) as a precursor for biologically active polyphosphazenes: Synthesis, characterization, and stabilization . In: Macromolecules . tape 37 , no. 2 , 2004, p. 414-420 , doi : 10.1021 / ma0355655 .
- ↑ Patent US3231327 : Preparation of N-dichlorophosphinyl-imidophosphoric trichloride. Filed November 13, 1961 , published January 25, 1966 , Applicant: FMC Corp., Inventor: L. Seglin, MR Lutz, H. Stange.
- ↑ R. De Jaeger, P. Potin: Chapter 2: Poly (dichlorophosphazene) from P-trichloro-N-dichlorophosphoryl monophosphazene Cl 3 P = N-POCl 2 , in Synthesis and Characterization of Poly (organophosphazenes) . Ed .: M. Gleria, R. De Jaeger. Nova Science Publishers, 2004, ISBN 1-59454-024-1 , pp. 25-48 .
- ↑ Patent US5132389 : Polycondensation of impure P 2 NOCl 5 into uncrosslinked poly (dichlorophosphazenes) in the presence of PCl5. Applied October 22, 1990 , published July 21, 1992 , Applicant: Atochem, Inventors: R. de Jaeger, G. D'Halluin, G. Pagniez, P. Potin.
- ↑ Patent US4198381 : Process for preparing low molecular weight linear phosphonitrilic chloride oligomers. Filed on Aug. 21, 1978 , published on April 15, 1980 , Applicant: Ethyl Corp., inventor ED Horn Baker, HM Li.
- ↑ TL Evans, HR Allcock: Poly (difluorophosphazene): A new intermediate for the synthesis of poly (organophosphazenes) . In: J. Macromol. Sci., Chem. Band 16 , no. 1 , 1981, p. 409-423 , doi : 10.1080 / 00222338108082059 .
- ^ HR Allcock, DB Patterson, TL Evans: Synthesis of Open-Chain Poly (difluorophosphazene) and its Reactions with Alkoxides . In: Macromolecules . tape 12 , no. 2 , 1979, p. 172-177 , doi : 10.1021 / ma60068a002 .
- ↑ a b Patent US5698664 : Synthesis of polyphosphazenes with controlled molecular weight and polydispersity. Filed April 26, 1995 , published December 16, 1997 , Applicant: The Penn State Research Foundation, University of Toronto, Inventor: HR Allcock, CT Morrissey, I. Manners, CH Honeyman.
- ↑ HR Allcock, CA Crane, CT Morrissey, JM Nelson, SD Reeves, CH Honeyman: “Living” cationic polymerization of phosphoranimines as an ambient temperature route to polyphosphazenes with controlled molecular weight . In: Macromolecules . tape 29 , no. 24 , 1996, pp. 7740-7747 , doi : 10.1021 / ma960876j .
- ^ HR Allcock, R. Prange: Properties of Poly (phosphazene - siloxane) Block Copolymers Synthesized via Telechelic Polyphosphazenes and Polysiloxane Phosphoranimines . In: Macromolecules . tape 34 , no. 20 , 2001, p. 6858-6865 , doi : 10.1021 / ma010088g .
- ^ Y. Cui, X. Tang, X. Huang, Y. Chen: Synthesis of the star-shaped copolymer of epsilon-caprolactone and L-lactide from a cyclotriphosphazene . In: Biomacromolecules . tape 4 , no. 6 , 2003, p. 1491-1494 , doi : 10.1021 / bm034237 + .
- ^ RA Montague, K. Matyjaszewski: Synthesis of poly [bis (trifluoroethoxy) phosphazene under mild conditions using a fluoride initiator . In: J. Amer. Chem. Soc. tape 112 , no. 18 , 1990, pp. 6721-6723 , doi : 10.1021 / ja00174a047 .
- ^ RD Chapman, MF Welker, CR Kreutzberger: Polyalkoxyphosphazenes by room-temperature polymerization of an electronegative phosphoranimine monomer . In: J. Inorg. Organomet. Polym. tape 6 , no. 3 , 1996, p. 267-275 , doi : 10.1007 / BF01057751 .
- ^ HR Allcock, RL Kugel, KJ Valan: Phosphonitrilic compounds. VI. High molecular weight poly (alkoxy and aryloxy-phosphazene) . In: Inorg. Chem. Band 5 , no. 10 , 1966, pp. 1709-1715 , doi : 10.1021 / ic50044a016 .
- ^ HR Allcock, RL Kugel: Phosphonitrilic compounds. VII. High molecular weight poly (diaminophosphazenes) . In: Inorg. Chem. Band 5 , no. 10 , 1966, pp. 1716-1718 , doi : 10.1021 / ic50044a017 .
- ^ HR Allcock, CT-W. Chu: Reaction of Phenyllithium with Poly (dichlorophosphazene) . In: Macromolecules . tape 12 , no. 4 , 1979, p. 551-555 , doi : 10.1021 / ma60070a003 .
- ↑ RH Neilson, P. Wisian-Neilson: poly (alkyl / arylphosphazenes) and Their Precursors . In: Chem. Rev. Band 88 , no. 3 , 1988, pp. 541-562 , doi : 10.1021 / cr00085a005 .
- ↑ K. Matyjaszewski, M. Cypryk, J. Dauth, RA Montague, ML White: New synthetic routes towards polyphosphazenes . In: Makromol. Chem. Macromol. Sym. tape 54–55 , no. 1 , 1992, p. 13-30 , doi : 10.1002 / masy.19920540105 .
- ↑ K. Matyjaszewski, U. Franz, RA Montague, ML White: Synthesis of polyphosphazenes from phosphoranimines and phosphine azides . In: polymer . tape 35 , no. 23 , 1994, pp. 5005-5011 , doi : 10.1016 / 0032-3861 (94) 90656-4 .
- ↑ a b O. Nuyken, ST Pask: Ring-opening polymerization - An introductory review . In: Polymers . tape 5 , 2013, p. 361-403 , doi : 10.3390 / polym5020361 .
- ↑ a b M. Gleria, R. De Jaeger: Polyphosphazenes: A Worldwide Insight . Nova Science Publishers, 2004, ISBN 1-59454-024-1 .
- ↑ LS Nair, YM Khan, CT Laurencin: Polyphosphazenes , in Handbook of Fluoropolymer Science and Technology, Chapter 22 . Ed .: JO Hollinger. CRC Press, 2012, ISBN 978-1-4398-1256-3 , pp. 385-400 .
- ^ V. Chandrasekhar: Inorganic and Organometallic Polymers . Springer, 2005, ISBN 3-540-22574-9 , doi : 10.1007 / b137079 .
- ^ HR Allcock, NL Morozowich: Bioerodible polyphosphazenes and their medical potential . In: Polym. Chem. Band 3 , 2012, p. 578-590 , doi : 10.1039 / C1PY00468A .
- ^ HR Allcock, GS McDonnell, JL Desorcie: Synthesis of new polyphosphazene elastomers . In: Macromolecules . tape 23 , no. 17 , 1990, pp. 3873-3877 , doi : 10.1021 / ma00219a001 .
- ^ AL Weikel, DK Lee, NR Krogman, HR Allcock: Phase changes of poly (alkoxyphosphazenes), and their behavior in the presence of oligoisobutylene . In: Polym. Closely. Sci. tape 51 , 2011, p. 1693-1700 , doi : 10.1022 / pen.21623 .
- ↑ A. Singh, L. Steely, HR Allcock: Poly [bis (2,2,2-trifluoroethoxy) phosphazene] superhydrophobic nanofibers . In: Langmuir . tape 21 , no. 25 , 2005, pp. 11604-11607 , doi : 10.1021 / la052110v .
- ↑ H. Kawakami, S. Kanezaki, M. Sudo, M. Kanno, S. Nagaoka, S. Kubota: Biodegradation and Biocompatibility of Polyorganophosphazene . In: Artificial Organs . tape 26 , 2002, p. 883-890 , doi : 10.1046 / j.1525-1594.2002.07029.x .
- ↑ a b H.R. Allcock: Fluorinated Polyphosphazenes , in Handbook of Fluoropolymer Science and Technology, Chapter 1 . Eds .: DW Smith, Jr., ST Iacono, SS Iyer. John Wiley & Sons, Inc., 2014, ISBN 978-0-470-07993-5 , pp. 1-20 .
- ^ HR Allcock, NJ Sunderland, R. Ravikiran, JM Nelson: Polyphosphazenes with Novel Architectures: Influence on Physical Properties and Behavior as Solid Polymer Electrolytes . In: Macromolecules . tape 31 , no. 23 , 1998, pp. 8026-8035 , doi : 10.1021 / ma9804491 .
- ↑ S. Jankowsky, MM Hiller, H.-D. Wiemhöfer: Preparation and electrochemical performance of polyphosphazene based salt-in-polymer electrolyte membranes for lithium ion batteries . In: J. Power Sources . tape 253 , 2014, p. 256–262 , doi : 10.1016 / j.powsour.2013.11.120 .
- ^ X. Zhou, J. Weston, E. Chalkova, MA Hofmann, CM Ambler, HR Allcock, SN Lvov: High temperature transport properties of polyphosphazene membranes for direct methanol fuel cells . In: Electrochim. Acta . tape 48 , no. 14-16 , 2003, pp. 2173-2180 , doi : 10.1016 / S0013-4686 (03) 00201-9 .
- ^ R. Wycisk, PN Pintauro: Polyphosphazene membranes for fuel cells . In: Adv. Polym. Sci. tape 216 , 2008, pp. 157-183 , doi : 10.1007 / 12_2007_130 .
- ↑ CJ Orme, FF Stewart: Mixed gas hydrogen sulfide permeability and separation using supported polyphosphazene membranes . In: J. Membrane Sci. tape 253 , no. 1–2 , 2005, pp. 243-249 , doi : 10.1016 / j.memsci.2004.12.034 .
- ↑ FF Stewart, MK Harrup, TA Luther, CJ Orme, RP Lash: Formation of pervaporation membranes from polyphosphazenes having hydrophilic and hydrophobic pendant groups: Synthesis and characterization . In: J. Appl. Polym. Sci. tape 80 , 2001, p. 422-431 , doi : 10.1002 / 1097-4628 (20010418) 80: 3 <422 :: AID-APP1115> 3.0.CO; 2-H .
- ↑ HR Allcock, S. Kwon, GH Riding, RJ Fitzpatrick, JL Bennett: Hydrophilic polyphosphazenes as hydrogels: radiation cross-linking and hydrogel characteristics of poly [bis (methoxyethoxyethoxy) phosphazene] . In: Biomaterials . tape 9 , no. 6 , 1988, pp. 509-513 , doi : 10.1016 / 0142-9612 (88) 90046-4 .
- ^ HR Allcock: Crosslinking reactions for the conversion of polyphosphazenes into useful materials . In: Chem. Mater. tape 6 , no. 9 , 1994, pp. 1476-1491 , doi : 10.1021 / cm00045a003 .
- ↑ a b Patent US8075916B2 : Poly (organophosphazene) hydrogels for drug delivery, preparation method thereof and use thereof. Filed June 14, 2007 , published December 13, 2011 , applicant: KIST Korea Institute of Science and Technology, inventor: S.-C. Song, M.-R. Park, S.-M. Lee.
- ^ Z. Huang, X. Liu, S. Chen, Q. Lu, G. Sun: Injectable and cross-linkable polyphosphazene hydrogels for space-filling scaffolds . In: Polym. Chem. Band 6 , 2015, p. 143-149 , doi : 10.1039 / C4PY00967C .
- ↑ a b A.K. Andrianov, S. Cohen, KB Visscher, LG Payne, HR Allcock, R. Langer: Controlled release using ionotropic polyphosphazene hydrogels . In: J. Control. Release . tape 27 , no. 1 , 1993, p. 69-77 , doi : 10.1016 / 0168-3659 (93) 90058-D .
- ^ HR Allcock, N. Morozowich: Bioerodible polyphosphazenes and their medical potential . In: Polym. Chem. Band 3 , 2012, p. 578-590 , doi : 10.1039 / C1PY00468A .
- ^ I. Teasdale, O. Brüggemann: Polyphosphazenes for medical applications . Smithers Rapra Technology, 2014, ISBN 978-1-909030-88-6 .
- ^ I. Teasdale, S. Wilfert, I. Nischang, O. Brüggemann: Multifunctional and biodegradable polyphosphazenes for use as macromolecular anti-cancer drug carriers . In: Polym. Chem. Band 2 , 2011, p. 828-834 , doi : 10.1039 / C0PY00321B .
- ^ I. Teasdale, O. Brüggemann: Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery . In: Polymers . tape 5 , 2013, p. 161-187 , doi : 10.3390 / polym5010161 .
- ↑ AL Baillargeon, K. Mequanint: Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics . In: BioMed Res. Int. 2014 . Article ID 761373, doi : 10.1155 / 2014/761373 .
- ↑ M. Deng et al. a .: Dipeptide-based polyphosphazene and polyester blends for bone tissue engineering . In: Biomaterials . tape 31 , no. 18 , 2010, p. 4898-4908 , doi : 10.1016 / j.biomaterials.2010.02.058 .
- ↑ NL Morozowich, JL Nichol, HR Allcock: Investigation of apatite mineralization on antioxidant polyphosphazenes for bone tissue engineering . In: Chem. Mater. tape 24 , no. 17 , 2012, p. 3500-3509 , doi : 10.1021 / cm3022825 .
- ↑ M. Deng, SG Kumar, Y. Wan, US Toti, HR Allcock, CT Laurencin: Polyphosphazene polymers for tissue engineering: an analysis of material synthesis, characterization and applications . In: Soft Matter . tape 6 , 2010, p. 3119-3132 , doi : 10.1039 / B926402G .
- ↑ S. Rothemund et al. a .: Degradable glycine-based photo-polymerizable polyphosphazenes for use as scaffolds for tissue regeneration . In: Macromol. Biosci. 2014, p. 1–13 , doi : 10.1002 / mabi.201400390 .
- ↑ H. Rose: About a connection between phosphorus and nitrogen . In: Ann. Pharm. Band 11 , 1834, pp. 129-139 , doi : 10.1002 / jlac.18340110202 .
- ^ J. Liebig: Addendum by the editorial office . In: Ann. Pharm. Band 11 , 1834, pp. 139-150 , doi : 10.1002 / jlac.18340110202 .