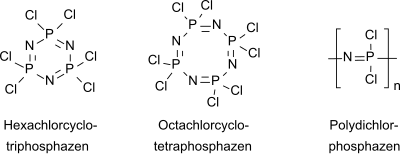
Hexachlorophosphazene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexachlorophosphazene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Cl 6 N 3 P 3 | |||||||||||||||
| Brief description |
light gray crystalline substance or white crystal powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 347.66 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.98 g cm −3 at 25 ° C |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
soluble in diethyl ether , benzene and toluene , |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexachlorophosphazene or phosphonitrile chloride trimer (NPCl 2 trimer) is an inorganic compound that exists as a six-membered ring with alternating phosphorus and nitrogen atoms with the empirical formula (NPCl 2 ) 3 .
Formally, hexachlorocyclotriphosphazene corresponds to the trimer of the hypothetical phosphonitrile chloride NPCl 2 . (NPCl 2 ) 3 reacts with water as an inorganic acid halide releasing hydrochloric acid and is therefore highly corrosive.
Like the homologous octachlorocyclotetraphosphazene (NPCl 2 ) 4, hexachlorocyclotriphosphazene is the starting compound for an extensive class of inorganic polymers, the polyphosphazenes .
Manufacturing
The synthesis of phosphorus nitrile chlorides (known as "chlorophosphorus nitrogen") from phosphorus pentachloride and gaseous ammonia is first mentioned in an exchange of letters by J. v. Liebig and F. Wöhler in 1832. The experiments on this were published in 1834.
The synthesis using ammonium chloride as a nitrogen source was described in 1870. The problem with the yield and purification of the product is already pointed out therein. Quotation: "The heating of phosphorus superchloride (PCl 5 ) and salmiak (NH 4 Cl) is recommended. The yield is very low. The substance can only be obtained completely pure and beautifully crystallized by sublimating".
The reaction in substance, i.e. That is, in the melt of phosphorus pentachloride (melting point: 166.8 ° C) cannot be controlled and gives only low yields (less than 15%) of hexachlorophosphazene even after several days of heating at temperatures above 200 ° C in closed apparatus. Under these reaction conditions, PCl 5 sublimes (sublimation temperature 160.5 ° C.) and ammonium chloride decomposes noticeably. Octachlorophosphazene (NPCl 2 ) 4 and an oil with the composition (NPCl 2 ) x are also obtained as by-products .
The low yields of cyclophosphazenes (38–43%) even with substantial (90–95%) conversion of the PCl 5 introduced make reactions in substance technically uninteresting.
The reaction is therefore usually carried out in halogenated aliphatic or aromatic solvents (for PCl 5 ) such as the toxic 1,1,2,2-tetrachloroethane, which is difficult to remove by distillation, or preferably in chlorobenzene with finely divided ammonium chloride suspended therein.
The yield of phosphonitrile chlorides in the heterogeneous reaction mixture depends not only on the reaction conditions (reaction medium, concentrations and addition of the reactants, mixing, temperature, reaction time, etc.), but also in particular on the particle size of the ammonium chloride used, on the surface of which the reaction takes place.
The reaction of the dissolved PCl 5 with the suspended NH 4 Cl takes place in a two-stage process, the insoluble salt Cl 3 P = N-PCl 3 + PCl 6 - further to Cl 3 P = N, which is formed rapidly and practically quantitatively in the first stage -PCl 2 = N-PCl 3 + PCl 6 - reacts and in the second step it reacts with ammonium chloride to form linear phosphonitrile chlorides (NPCl 2 ) n , which can then cyclize to the cyclophosphazenes.
The product obtained is a mixture of crystalline cyclic (trimer, tetramer, pentamer) and oily linear oligomeric phosphonitrile chlorides.
The stated product yields often relate to the crystalline cyclic fraction from which hexachlorophosphazene is isolated in pure substance by extraction and distillation or melt filtration or sublimation .
Numerous variants are described in the patent literature which aim to increase the proportion of the cyclic fraction in the product mixture and, in particular, the proportion of the trimeric phosphonitrile chloride (NPCl 2 ) 3 (hexachlorophosphazene). So z. B. PCl 5 reacted in HCl-saturated chlorobenzene with ammonia at 130 ° C for 4 to 5 hours and the total product is obtained in a yield (based on PCl 5 ) of 92%, which contains 65 to 75% cyclic phosphonitrile chlorides, of which 60 up to 75% of the trimer.
The binding of the hydrogen chloride formed in the reaction of PCl 5 with NH 4 Cl by inorganic, such as. B. zinc oxide and organic bases such as pyridine or triethylamine as acid scavengers, optionally with further addition of metal halides, accelerates the reaction and increases the product yields. Salt -like oligomers with the composition [Cl 3 P = N (PNCl 2 ) n -PCl 3 ] + PCl 6 - are also formed , which remove PCl 5 from the reaction to form the PNCl 2 trimer and suggest target product yields of over 100%.
A more recent patent describes the synthesis of NPCl 2 trimer by converting a phosphorus pentachloride-pyridine complex and reacting with finely divided ammonium chloride in chlorobenzene, after which a total yield of 98.6% with a proportion of 84.2% cyclic NPCl 2 trimer was achieved .
Because of the large number of products with the empirical formula (NPCl 2 ) n which are formed in the reaction of PCl 5 and NH 4 Cl and are difficult to handle because of their unpleasant properties, information on the yield (and often also purity) of the cyclic NPCl 2 trimer obtained is therefore provided to be critically assessed in the literature.
The separation of the (NPCl 2 ) 4 tetramers contained in the commercially available phosphonitrile chloride trimers is carried out, for. B. by recrystallization from n-hexane and subsequent vacuum sublimation at 60 ° C.
properties
When crystallized from benzene, hexachlorocyclotriphosphazene forms centimeter-sized prisms and from diethyl ether "magnificent, transparent, colorless leaves"
In many organic solvents, such as. B. aliphatic ( n-hexane ) and aromatic hydrocarbons ( benzene , toluene , benzene derivatives such as chlorobenzene , nitrobenzene , ethers, diethyl ether , tetrahydrofuran ) and in acetonitrile , hexachlorophosphazene is readily soluble. The compound has a relatively high vapor pressure, a "fairly pleasant organic odor" and is irritating to the eyes and respiratory organs.
In water, PNCl 2 trimer hydrolyzes with elimination of HCl to form oxophosphazenes and further with ring splitting to form phosphoric acid, ammonia and hydrochloric acid.
Hexachlorocyclotriphosphazene has a hexagonal planar ring structure with equidistant NP bonds which, at 158 pm, are significantly shorter than single NP bonds (177 pm). The bond angles are consistent with sp 2 hybridization of the nitrogen atoms and (approximately) sp 3 hybridization of the phosphorus atoms. In contrast to benzene, d and p orbitals are involved in the π bonds of cyclophosphazenes and cyclophosphazenes are also significantly more difficult to hydrogenate than the aromatic benzene.
Applications
The six chlorine atoms in hexachlorophosphazene can be successively replaced by nucleophilic substitution of amino, alkyl, aryl, alkoxy, aryloxy, alkylamino and arylamino groups, the place and degree of substitution being strongly dependent on the chosen reaction conditions and the charge distribution in the mesomeric cyclophosphazene Ring system and depend on steric and mechanistic effects.
Alkoxy and aryloxy derivatives of hexachlorophosphazene have been intensively investigated for their suitability as high-temperature-resistant and flame-retardant hydraulic fluids and lubricating oils . Mixed esters obtained in a one-pot reaction with trifluoroethanol , phenol , m-cresol and p-cresol with the approximate composition:
- N 3 P 3 (OCH 2 CF 3 ) 3.5 (OC 6 H 5 ) 1.25 (OC 6 H 4 -m-CH 3 ) 0.87 (OC 6 H 4 -p-CH 3 ) 0.38
With an aryloxy / fluoroalkoxy ratio of 2.5: 3.5, the requirements for viscosity , pour point , thermal and hydraulic stability and compatibility with plastics and painted surfaces are best .
The fluorine-analogous hexafluorocyclotriphosphazene (NPF 2 ) 3 is formed from NPCl 2 trimer and NaF at room temperature in acetonitrile as a solvent, while the bromine-analogous hexabromocyclophosphazene (NPBr 2 ) 3 such as (NPCl 2 ) 3 from phosphorus pentahalide , in this case from PBr 5 and NH 4 Br, is accessible.
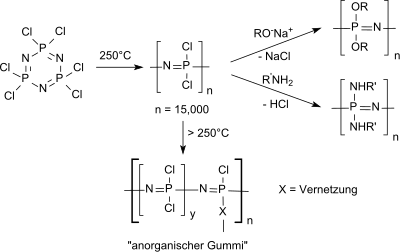
The most important use of hexachlorophosphazene is as a starting compound for linear polydichlorophosphazene (in crosslinked form often referred to as "inorganic rubber") and for functionalized polyphosphazenes ,
which, due to their exceptional properties, have a wide range of applications, such as B. in energy storage ( lithium ion batteries , in fuel cells ), in photonics (photoluminescence), as non-linear optical (NLO) materials, as liquid crystals , in photochromics , etc., as high-performance elastomers and as biomedical polymers.
Individual evidence
- ↑ a b c d Datasheet Phosphonitrile Chloride Trimer from Sigma-Aldrich , accessed on January 25, 2015 ( PDF ).
- ↑ a b c data sheet Phosphonitrilic chloride trimer at AlfaAesar, accessed on January 25, 2015 ( PDF )(JavaScript required) .
- ↑ Data sheet Phosphonitrilic chloride, trimer (PDF) from Fisher Scientific , accessed on May 25, 2017.
- ↑ a b Entry on Phosphonitrilic Chloride Trimer at TCI Europe, accessed on January 25, 2015.
- ↑ a b c H.N. Stokes: On the chloronitriles of phosphorus . In: Amer. Chem. J. Volume 17 , 1895, p. 275-290 ( online ).
- ^ JE Mark, HR Allcock, R. West: Polyphosphazene , in Inorganic Polymers, Second Edition . Oxford University Press, 2005, ISBN 0-19-513119-3 , pp. 62-153 .
- ^ AW Hofmann (Ed.): From Justus Liebig and Friedrich Wöhler's correspondence in the years 1829–1873 . Vieweg, 1832, p. 63 .
- ↑ H. Rose: About a connection between phosphorus and nitrogen . In: Ann. Pharm. Band 11 , 1834, pp. 129-139 , doi : 10.1002 / jlac.18340110202 .
- ^ J. Liebig: Addendum by the editorial office . In: Ann. Pharm. Band 11 , 1834, pp. 139-150 , doi : 10.1002 / jlac.18340110202 .
- ↑ H. Wichelhaus: About chlorophosphorus nitrogen . In: Ber. German Chem. Ges. Volume 3 , 1870, p. 163-166 , doi : 10.1002 / cber.18700030155 .
- ↑ HN Stokes: About chlorophosphorus nitrogen and two of its homologous compounds . In: Ber. German Chem. Ges. Volume 28 , 1895, p. 437-439 , doi : 10.1002 / cber.189502801106 .
- ^ R. Steinman, FB Schirmer, Jr., LF Audrieth: The preparation and physical properties of trimeric phosphonitrilic chloride . In: J. Amer. Chem. Soc. tape 64 , no. 10 , 1942, pp. 2377-2378 , doi : 10.1021 / ja01262a044 .
- ↑ a b c R. Schenck, G. Römer: About the phosphonitrile chlorides and their reactions (I.) . In: Ber. dtsch. Chem. Ges. A / B . tape 57 , no. 8 , 1924, pp. 1343-1355 , doi : 10.1002 / cber.19240570823 .
- ↑ a b H.R. Allcock: Phosphorus-Nitrogen Compounds: Cyclic, Linear and High Polymeric Systems, Chapter 4: Synthesis of the phosphorus-nitrogen skeleton . Academic Press, 1972, ISBN 978-0-12-050560-9 , pp. 97-133 .
- ↑ M. Becke-Goehring, E. Fluck: The way from phosphorus pentachloride to the phosphorus nitrile chlorides . In: Angew. Chem. Band 74 , no. 11 , 1962, pp. 382-386 , doi : 10.1002 / anie.19620741104 .
- ↑ J. Emsley, PB Udy: Elucidation of the reaction of phosphorus pentachloride and ammonium chloride by phosphorus-31 nuclear magnetic resonance spectroscopy . In: J. Chem. Soc. A . 1970, p. 3025-3029 , doi : 10.1039 / J19700003025 .
- ↑ Patent US4605539 : Phosphonitrile chloride trimer purification. Filed November 16, 1984 , published August 12, 1986 , Applicant: Ethyl Corp., Inventor: JR Adams Jr., JR Mitrano, MK Juneau.
- ↑ Patent EP0185173A2 : Method for purifying cyclic hexachlorophazene trimer for catalytic impurities. Filed October 28, 1985 , published June 25, 1986 , Applicant: The Firestone Tire & Rubber Co., Inventor: DF Graves, DL Snyder.
- ↑ Patent DE2328536 : Process for the production of phosphonitrile chloride. Filed June 5, 1973 , published December 20, 1973 , Applicant: Ethyl Corp., Inventor: CR Bergeron, JT-F. Kao.
- ↑ Patent US4567028 : Process for the preparation of phosphonitrile chloride oligomer. Applied on February 11, 1985 , published on January 28, 1986 , Applicants: Shin Nisso Kako Co., Ltd., Inventors: H. Tanino, T. Okamoto, S. Ueyama.
- ↑ Patent US4656017 : Pyridine phosphonitrilic halide process. Filed on July 19, 1985 , published on April 7, 1987 , Applicant: Ethyl Corp., inventor HR Allcock, SJ Stinnett, JB Tedder, Jr., JR Adams, Jr
- ↑ R. Koci Voznicova, J. Taraba, J. Prihoda, M. Alberti: The synthesis and characterization of new aminoadamantane derivatives of hexachloro-cyclo-triphosphazene . In: Polyhedron . tape 27 , 2008, p. 2077-2082 , doi : 10.1016 / j.poly.2008.04.001 .
- ↑ N.-N. Tian, L.-S. Wang, M.-Y. Li, Y. Li, R.-Y. Jiang: Solubilities of Phenylphosphinic Acid, Methylphenylphosphinic Acid, Hexachlorocyclotriphosphazene, and Hexaphenoxycyclotriphosphazene in Selected Solvents . In: J. Chem. Data . tape 56 , no. 3 , 2011, p. 661-670 , doi : 10.1021 / je1009812 .
- ^ FF Stewart, ES Peterson: Handbook of Ring-Opening Polymerization, Chapter 4: Sulfur-Nitrogen-Phosphorus-Containing Polymers . Ed .: P. Dubois, O. Coulembier, J.-M. Raquez. Wiley-VCH, 2009, ISBN 978-3-527-31953-4 , pp. 97-122 .
- ↑ GR Feistel, MK Feldt, RL Dieck, T. Moeller: Inorganic Syntheses, Volume XIV . Mc Graw-Hill, 1973, Chapter One: Phosphorus Compounds , p. 23-27 , doi : 10.1002 / 9780470132456 .
- ^ S. Ganapathiappan, SS Krishnamurty: Studies of phosphazenes. Part 30. Reactions of hexachlorocyclotriphosphazene with aromatic primary amines: interplay of geminal and non-geminal modes of chlorine replacement . In: J. Chem. Soc., Dalton Trans. 1987, pp. 579-584 , doi : 10.1039 / DT9870000579 .
- ^ CW Allen: Regio- and stereochemical control in substitution reactions of cyclophosphazenes . In: Chem. Rev. Band 91 , no. 2 , 1991, p. 119-135 , doi : 10.1021 / cr00002a002 .
- ↑ RE Singler, FJ Gomba: Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology, 2nd. Edit., Chapter 14: Phosphazenes . Ed .: LR Rudnick. CRC Press, 2013, ISBN 978-1-4398-5537-9 , pp. 235-244 .
- ↑ Patent US20140178752A1 : Cyclotriphosphazene compound, method of preparing the same, electrolyte for lithium secondary battery including the cyclotriphosphazene compound, and lithium secondary battery including the electrolyte. Filed December 24, 2013 , published June 26, 2014 , applicant: Samsung Sdi Co., Ltd., inventor: YS Park, H.-S. Yang, Y.-H. Kim, J.-H. Lim, H.-Y. Hwang.
- ^ The Pennsylvania State University, Applications , online