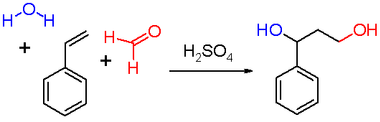
Prins reaction
The Prins reaction is an organic chemistry reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne , followed by the addition of a nucleophile to the resulting intermediate. The outcome of the reaction depends essentially on the reaction conditions ( see scheme 1 ). The reaction product of an alkene with formaldehyde in water or in the presence of a Brønsted acid is a 1,3-diol , while with the exclusion of protic solvents, water is split off to give the corresponding allyl alcohol . If there is an excess of formaldehyde and the reaction temperature is low, the product is an acetal with formaldehyde (1,3-dioxane) ; if the reaction takes place in acetic acid as solvent, the corresponding carboxylic acid ester is formed.
history
The reaction was originally published by the Dutch chemist Hendrik Jacobus Prins (1889–1958) in 1919 as a reaction of styrene ( see Scheme 2 ), pinene , camphor , eugenol , isosafrole and anethole with formaldehyde:
In 1937, the reaction became important as part of a synthesis for diolefins, for the production of isoprene and thus also synthetic rubber (with the structure of natural rubber ):
Reaction mechanism
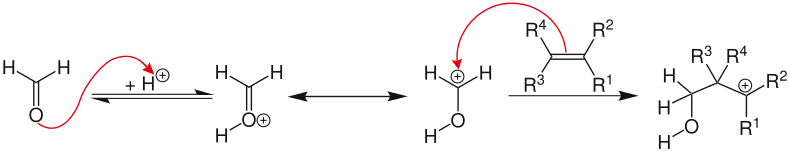
First, the carbonyl compound is protonated by the acid, so that the oxonium ion results. The electrophile attacks the alkene in an electrophilic addition , resulting in the carbocation intermediate. There are indications that this is stabilized via a neighboring group effect.
There are then a total of four possible reaction pathways for the resulting oxo- carbocation intermediate:
- The carbocation is captured by water or another suitable nucleophile and reacts to form the 1,3-adduct.
- The carbocation is intercepted by another carbonyl compound. In this way, the positive charge is delocalized over oxygen and carbon in mesomeric limit formulas. Dioxane is formed in a ring closure . One example is the conversion of styrene to 4-phenyl-m-dioxane.
- It starts an elimination reaction to form an unsaturated compound. If the alkene has a methyl group, the elimination can be followed by the transfer of an allyl proton and then the addition of a carbonyl group.
- The oxetane is only formed in very special cases and only when the carbocation formed as an intermediate is extremely stable . The photochemically induced Paternò - Büchi reaction between alkenes and aldehydes to form oxetanes is, however, generally less complicated.
variants
There are a number of variations for the Prins reaction, as it can be used to form rings on the one hand and a wide range of nucleophiles to trap the intermediate oxo-carbenium ion on the other.
Halo Prins reaction
The Halo-Prins reaction is one of these modifications: Protic solvents or Brønsted acids are replaced by Lewis acids such as tin (IV) chloride , titanium (IV) chloride or boron tribromide . The halogen now represents the nucleophile that recombines with the carbocation. The ring closure of the allyl pulegone in Scheme 7 with titanium tetrachloride in dichloromethane at −78 ° C is thus a possible diastereoselective route to the synthesis of a decalin structure with a hydroxyl and chloride group in the cis position ( de = 91% cis ). The cause is the intermediate formation of the trichlorotitanium alkoxide, on which the addition of the chloride to the carbocation takes place preferably from the same side. The trans diastereomer is preferably formed when the reaction is carried out in tin tetrachloride at room temperature.
Prins pinacol reaction
The Prins-pinacol reaction is a cascade reaction consisting of a Prins reaction and a pinacol rearrangement . The carbonyl group in Scheme 8 is used as a protected dimethyl acetal and the hydroxy group as a triisopropylsilyl ether (TIPS). The oxonium ion is activated with the Lewis acid tin chloride and the pinacol rearrangement of the intermediate of the Prins reaction leads to a reduction in the size of the ring with a shift in the positive charge to the TIPS ether, which decomposes into an aldehyde group with moderate diastereoselectivity .
Individual evidence
- ^ HJ Prins: Condensation of formaldehyde with some unsaturated compounds, Chemisch Weekblad 1919 , 16, 64, 1072, 1510.
- ↑ Chemical Abstracts , 1919 13 , 3155.
- ↑ E. Arundale, LA Mikeska: The Olefin-Aldehyde Condensation. The Prins Reaction , Chem. Rev .; 1952 ; 51 (3) ; 505-555.
- ^ RL Shriner and Philip R. Ruby: 4-Phenyl-m-dioxane In: Organic Syntheses . 33, 1953, p. 72, doi : 10.15227 / orgsyn.033.0072 ; Coll. Vol. 4, 1963, p. 786 ( PDF ).
- ^ R. Brandon Miles, Chad E. Davis, and Robert M. Coates: Syn- and Anti-Selective Prins Cyclizations of, -Unsaturated Ketones to 1,3-Halohydrins with Lewis Acids , J. Org. Chem. 2006 , 71 , 1493-1501.
- ↑ Larry E. Overman and Emile J. Velthuisen: Scope and Facial Selectivity of the Prins-Pinacol Synthesis of Attached Rings , J. Org. Chem. 2006 , 71 , 1581-1587.