Isosafrole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| ( E , Z ) isomerism undefined | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Isosafrole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 10 O 2 | |||||||||||||||
| Brief description |
colorless liquid, aniseed smell |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 162.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.573 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Isosafrole is an organic compound from the group of phenylpropanoids .
Isomerism
Because of the double bond isomerism at the noncyclic C = C double bond, there are two forms of isosafrole, ( Z ) -isosafrole (also known as cis- or α-isosafrole) and ( E ) -isosafrole ( trans- or β-isosafrole), which more stable shape.
| Isomers of isosafrole | ||||
| Surname | ( Z ) -Isosafrole | ( E ) -Isosafrole | ||
| other names |
cis -isosafrole α-isosafrole |
trans -isosafrole β-isosafrole |
||
| Structural formula |

|

|
||
| CAS number | 17627-76-8 | 4043-71-4 | ||
| EC number | 241-611-4 | 204-410-2 | ||
| ECHA info card | 100,037,813 | 100.004.010 | ||
| PubChem | 1549044 | 637796 | ||
| Wikidata | Q27253905 | Q1088741 | ||
Occurrence
Safrole and isosafrole are found in essential oils of various lauraceae and myristicaceae . The cis form is sometimes found in traces in ylang-ylang oil .
Extraction and presentation
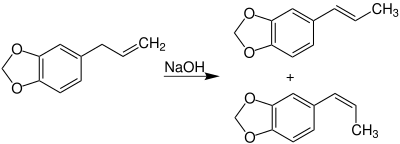
It is produced by the alkaline isomerization of safrole using sodium hydroxide (at room temperature under pressure):
Alternatively, the isomerization of safrole to isosafrole can also take place with alcoholic potassium hydroxide solution at boiling temperature.
proof
With the help of the Chapman reaction , a safrole solution can be distinguished from an isosafrole solution. A solution is prepared from 1 part isosafrole and 5 parts acetic anhydride and then 1 drop of concentrated sulfuric acid is added, the color of the solution being pink to reddish. Adding dissolved zinc chloride changes the color from pink to brown. If this reaction is carried out with safrole instead of isosafrole, the color change is either from emerald green to brown or, if dissolved zinc chloride is added, from pink to brown.
properties
Isosafrole is an almost colorless oil with a weaker odor than safrole. It has an aniseed or liquorice smell .
use
The substance is used in small quantities in the manufacture of perfumes and other cosmetics. In the United States, the substance has long been used as a flavoring in root beer . As a raw material for the synthesis of MDMA (Ecstasy), isosafrole falls under the Basic Substance Monitoring Act (GÜG). Accordingly, the transport quantities must be logged and the loss of the chemical must be reported.
Individual evidence
- ↑ a b c M. Bergmann, K. Boresch, R. Brieger: Handbuch der Pflanzenanalyse . Special analysis: Organic substances II. Springer-Verlag, 1932, ISBN 978-3-7091-2230-3 , p. 528 ( Google Books ).
- ↑ a b c d Wolfgang Legrum: Fragrances, between stench and fragrance: Occurrence, properties and use of fragrances and their mixtures . Springer-Verlag, 2015, ISBN 978-3-658-07310-7 , pp. 132 ( Google Books ).
- ↑ J. Heck, B. König & R. Winter (eds.): Fragrances, between stench and fragrance. Wolfgang Legrum, Springer Spectrum, Marburg 2013, ISBN 978-3-658-07310-7 , e-book, p. 132.
- ↑ a b c d e f data sheet Isosafrol, mixture of cis and trans at Sigma-Aldrich , accessed on October 21, 2017 ( PDF ).
- ↑ a b Richard J. Lewis: Hazardous Chemicals Desk Reference . John Wiley & Sons, 2008, ISBN 978-0-470-33445-4 , pp. 805 ( Google Books ).
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 ( Google Books ).
- ^ A b Paul Hoering, Reports of the German Chemical Society 42 (1909) pp. 3076-3088.
- ↑ a b Hazardous Substance Fact Sheet: 1,2-METHYLENEDIOXY-4-PROPENYL-BENZENE. (PDF) New Jersey Department of Health and Senior Services, January 2001, accessed October 19, 2017 .
- ↑ Benzene, 1,2- (methylenedioxy) -4-propenyl-. In: Registry of Toxic Effects of Chemical Substances . National Institute for Occupational Safety and Health , accessed October 19, 2017 .
- ↑ Essential Oil Safety - E-Book: A Guide for Health Care Professionals . Elsevier Health Sciences, 2013, ISBN 978-0-7020-5434-1 , pp. 579 ( Google Books ).
- ↑ Fred Winter: Handbook of the entire perfumery and cosmetics . Springer-Verlag, 1932, ISBN 978-3-662-02107-1 , p. 107 ( Google Books ).
- ↑ E. Merck (Ed.): Merck's Reagenzien -verzeichnis - containing the common reagents and reactions, sorted by author name . Julius Springer, Berlin, ed. 4, 1916, ISBN 978-3-662-42388-2 , p. 73, doi : 10.1007 / 978-3-662-42388-2 .