Propylhexedrine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| ( R ) -isomer (left) and ( S ) -isomer (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Propylhexedrine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 21 N | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 155.28 g mol −1 | ||||||||||||||||||
| density |
0.85 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
|
||||||||||||||||||
| pK s value |
4.78 (pK b ) |
||||||||||||||||||
| solubility |
Slightly soluble in water, miscible with ethanol , chloroform and diethyl ether |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Propylhexedrine (also hexahydrodeoxyephedrine) is a central nervous system stimulant marketed in the United States as the Benzedrex inhaler pen . Propylhexedrine is structurally related to methamphetamine .
It is mainly used for the symptomatic treatment of nasal discharge from colds and allergies .
The ( S ) enantiomer , also known as levopropylhexedrine , is also used as an appetite suppressant . The salt of the ( S ) -enantiomer with phenobarbital gives the antiepileptic drug barbexaclone .
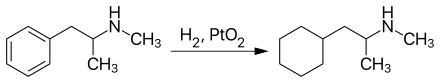
synthesis
Propylhexedrine can be produced by the nuclear hydrogenation of methamphetamine in the presence of platinum (IV) oxide as a catalyst :
An enantiomer separation can with the help of L - tartaric acid - (+) take place.
Individual evidence
- ↑ a b c d The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals . 14th edition. Merck & Co., Whitehouse Station NJ 2006, ISBN 978-0-911910-00-1 , pp. 1350-1351.
- ↑ a b c Entry on levopropylhexedrine. In: Römpp Online . Georg Thieme Verlag, accessed on November 15, 2014.
- ^ LG Chatten, LE Harris: Relationship between pKb (H2O) of Organic Compounds and E 1∕2 Values in Several Nonaqueous Solvents. , in: Anal. Chem. 1962 , 34 , 1495-1501; doi : 10.1021 / ac60191a041 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications, 4th Edition, Thieme 2001, ISBN 3-13-115134-X .
- ↑ Lands, AM; Nash, VL; Granger, HR; Dertinger, BL: The Pharmacologic Activity of N-Methyl-β-cyclohexylisopropylamine Hydrochloride in J. Pharmacol. Exp. Ther. 89 (1947) 382-385.
- ↑ External identifiers or database links for levopropylhexedrine : CAS number: 6192-97-8, EC number: 228-245-0, ECHA InfoCard: 100.025.678 , PubChem : 71197 , ChemSpider : 64333 , Wikidata : Q15409406 .
- ↑ BL Zenitz, EB Macks, ML Moore: Preparation of Some Primary and Secondary β-Cyclohexylalkylamines in J. Am. Chem. Soc. 69 (1947) 1117-1121. doi : 10.1021 / ja01197a039
- ↑ Patent US2454746 : Cyclohexylalkylamines. Filed May 13, 1947 , published November 23, 1948 , Applicant: Smith, Kline & French Labor, Inventor: Glenn E. Ullyot.