Rigid rotator
The rigid rotator is an ideal model of mechanics , which is used to describe rotating bodies . In this model, two or more mass points of a body rotate at a fixed distance around a main axis of inertia . For a description in three dimensional space normally three different principal axes of inertia are the moments of inertia , , defines which usually increase as a convention in the mentioned order.
Linear rigid rotator
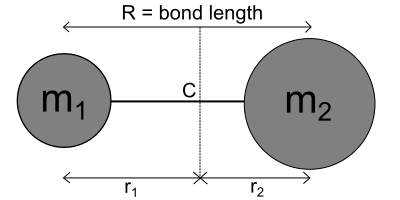
With the linear rigid rotator, the body consists of two or more linearly arranged mass points, each of which has a fixed distance from the center of mass. The axis of rotation runs through the center of mass. Examples of such structured molecules are the hydrogen molecule or carbon dioxide . The moment of inertia for this rotation is classically calculated using the equation
whereby instead of the sum of the mass points with the respective distances to the axis of rotation, the reduced mass with the distance between the two masses is used.
A special feature of the linear rigid rotator is that the moment of inertia of the main axis of inertia is zero. From this it follows that this rotator can be described with the two identical moments of inertia .
Classic calculation of energy
In a classic viewing of the rigid rotor corresponding to the total energy of the rotational energy of the system . This is related to the angular velocity around the main axis of inertia moment of inertia by
together.
Quantum mechanical calculation of energy
In quantum mechanics , the rigid rotator is described by a wave function . Its energy eigenvalues result from the solution of the stationary Schrödinger equation
- .
Since the particle is not exposed to any electrical potential and has only kinetic energy , the Hamilton operator reads:
Here corresponds to the Laplace operator . This is in spherical coordinates as
defined, whereby the first term is omitted due to the constant distance. Therefore, a new operator can be used as
To be defined. By describing the Schrödinger equation in spherical coordinates instead of Cartesian coordinates , it can be divided into two functions. The former is only dependent on, while the spherical surface function depends on both the polar angle and the azimuth angle . By inserting the aforementioned equations into the Schrödinger equation, one obtains:
The application of the abbreviated Laplace operator to the spherical surface function returns this multiplied by the factor .
It follows from this that the permitted rotational energy levels are based on the formula
After introducing the constant of rotation as
and substitution of the rotational quantum number can be the more common form
are written, resulting in the spectroscopy usual rotation terms by
can be calculated.
Spherical rotator
A spherical rotator exists when the moments of inertia of the main axes of inertia are identical and thus . From this it can be concluded that the rotational energy levels can be calculated analogously to the linear rotator. An example of such a rotator is the methane molecule . Since there is no permanent dipole moment in this rotator , transitions in the rotation levels only occur due to centrifugal expansion of the molecule, as this induces a permanent dipole moment (see section on selection rules ).
Centrifugal expansion
A customary adaptation of the rigid rotator model to describe real systems is to allow the distances to be stretched by the centrifugal force occurring during rotation . The correction is usually made by introducing a strain constant that depends on . This gives the permitted rotational energy levels from now on through the equation
The expansion constant for diatomic molecules can be derived from the rotation constant and the wave number of the fundamental oscillation according to the equation
to be determined.
Symmetrical rotator
Symmetrical rotators are characterized by the fact that the moments of inertia of two main axes of inertia are identical. In the case of one speaks of a flat rotator and in the case of a pointed symmetrical rotator. Since only two main moments of inertia are identical, a further rotational quantum number is required for the rotation terms analogous to that in the spherical surface function.
In the case of a pointed symmetrical rotator, the constant of rotation corresponds to and , while in the case of a flat symmetrical rotator corresponds to the constant of rotation . The energy levels of a symmetrical rotator can be obtained from the rotation terms or, analogously to the linear rotator, by applying the Hamilton operator to the Schrödinger equation. This leads to the solution
for the pointed respectively
for the flat symmetrical rotator.
Asymmetrical rotator
In the asymmetrical rotator, the moments of inertia of all main axes of inertia differ from one another. This leads to the fact that there are no generally valid formulas for calculating the energy levels in quantum mechanical considerations, but exact formulas exist for certain rotational quantum numbers. Classically, the energy can still be calculated using the main moments of inertia and the angular speeds.
Selection rules
The transitions in the energy levels of a rigid rotator follow certain rules, the so-called selection rules. The change of the rotational energy level by emission or absorption of electromagnetic radiation is always allowed if the transition dipole moment of the transition according to the equation
does not go away. The equation can be simplified for pure rotational transitions, as they are the only ones possible in the rigid rotator, after integration via the vibronic functional components
It follows from this that rotational transitions due to electromagnetic radiation are only permitted for molecules with a permanent dipole moment, i.e. molecules in which the integral does not vanish. The hereafter allowed transitions contact for functions with opposite order
changed quantum numbers on what the changes of the rotational quantum numbers
corresponds. These rules are called selection rules.
literature
- Gerd Wedler , Hans-Joachim Freud: Textbook of physical chemistry . 6th edition. Wiley-VCH, Weinheim 2013, ISBN 978-3-527-32909-0 , pp. 532-540 .
- Peter Atkins , Julio de Paula: Physical Chemistry . 10th edition. Oxford University Press, 2014, ISBN 978-1-4292-9019-7 , 12B-12C, pp. 488-498 .
- Kurt W. Kolasinski: Physical chemistry . 1st edition. Wiley, New York 2017, ISBN 978-1-118-75112-1 , 25.2, pp. 604-608 .
- Reiner Salzer, Steffen Thiele, Lydia Suemmchen: Rotational vibration spectra. In: chemgapedia.de. Wiley-VCH, accessed July 13, 2018 .
Individual evidence
- ↑ P. D. Jarvis, L. A. Yates: The molecular asymmetric rigid rotor Hamiltonian as an exactly solvable model . In: Molecular Physics . tape 106 , no. 7 , 2008, doi : 10.1080 / 00268970802050394 , arxiv : 0803.2562 .





















![{\ displaystyle {\ hat {o}} = \ left [{\ frac {1} {r ^ {2} \ sin (\ theta)}} \ cdot {\ frac {\ partial} {\ partial \ theta}} \ left (\ sin (\ theta) \ cdot {\ frac {\ partial} {\ partial \ theta}} \ right) + {\ frac {1} {r ^ {2} \ sin ^ {2} (\ theta )}} \ cdot {\ frac {\ partial ^ {2}} {\ partial \ phi ^ {2}}} \ right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a47a113c0111c0f0f37a395f4b2027bfef285935)









































