Takeda olefination
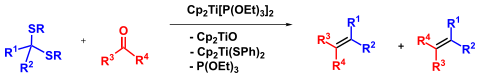
The Takeda olefination is a titanium -mediated chemical reaction for the olefination of dithioacetals (and ketals ) and carbonyl compounds ( aldehydes , ketones or esters ), which was published by Takeshi Takeda in 1997. It is considered a comparatively mild, non-basic olefination for sensitive starting materials for the preparation of di-, tri- and even tetrasubstituted olefins.
Coupling reaction
Since the thioacetals can be prepared from carbonyl compounds, the Takeda olefination can also be viewed as a coupling reaction between two carbonyl compounds, similar to the McMurry coupling . Instead of dithioacetals, geminal dihalides can also be used to olefine carbonyl groups, which is strongly reminiscent of the Takai and Takai-Lombardo reactions.
mechanism
The Takeda olefination presumably takes place via Ti- carbene species . These Ti carbenes react with carbonyl groups via four-membered oxametallacyclobutanes. This mechanism is reminiscent of the intermediate oxaphosphetane of the Wittig reactions , but is more closely related to olefin metathesis due to the metal carbene intermediate . If prepared in the presence of olefins, this can lead to cyclopropanation of the double bond, as with the related Rh carbenes .
A dititanium species analogous to the Takai reaction was proposed as an alternative mechanism.
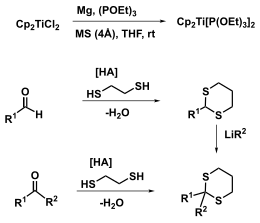
Representation of the starting materials
The Titanocenbis (triethyl phosphite) can be achieved by reduction of titanocene dichloride Cp 2 Ti (IV) Cl 2 with magnesium in the presence of triethyl phosphite and molecular sieve represent.
Dithioacetals and ketals can easily be prepared from aldehydes and ketones by reaction with thiols or dithiols. Alternatively, thioketals can arise from the thioacetals ( Corey-Seebach ). Thioketals are often used as protecting groups , which leads to many points of contact for the Takeda olefination in total synthesis .
Comparison with other methods
In general, titanium-mediated olefinations ( Tebbe , Petasis , Takai , Lombardo , McMurry ) take place with a range of carbonyl compounds (aldehydes, ketones, esters, lactones, amides), whereas other olefinations ( Wittig , Julia , Peterson ) mostly use aldehydes and ketones are limited. In addition, titanium-mediated olefinations are comparatively mild and tolerate a number of functional groups as well as easily enolizable alpha-chiral carbonyl compounds, since these reactions take place under non-basic conditions. On the other hand, the practical use of Ti-based olefinations is often limited to methenylations (Tebbe, Petasis, Takai methylenation, Takai-Lombardo methylenation). The extensions of these methods (Takai-Utimoto and Takai-Lombardo olefination) require geminal dihalides or their analogues as reaction partners, the preparation of which has so far been rather laborious.
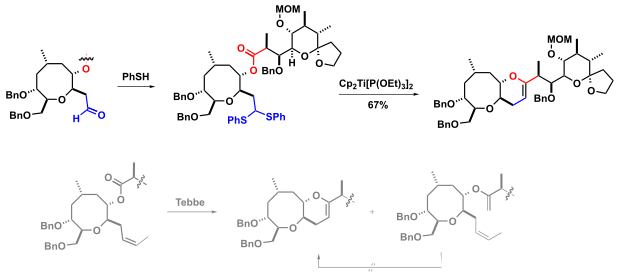
In the total synthesis of natural products are u. a. intramolecular versions of the Takeda reaction are popular because they allow the regioselectivity (E / Z selectivity) of the Takeda olefination to be geometrically enforced.
Hirama and co-workers use Takeda olefination in the synthesis of Ciguatoxin CTX3C. The authors' original synthesis plan instead called for a Tebbe methylenation with subsequent olefin metathesis (RCM). However, the Tebbe reaction provided a mixture of products and the planned ring-closing metathesis of the bis-olefin was unsuccessful.
Individual evidence
- ↑ a b c Yasuo Horikawa, Mikako Watanabe, Tooru Fujiwara, Takeshi Takeda: New Carbonyl Olefination Using Thioacetals . In: Journal of the American Chemical Society . tape 119 , no. 5 , February 1997, ISSN 0002-7863 , p. 1127-1128 , doi : 10.1021 / ja962240d .
- ↑ Takeshi Takeda: Organic Syntheses Utilizing Titanium Carbene Complexes . In: Bulletin of the Chemical Society of Japan . tape 78 , no. 2 , February 2005, ISSN 0009-2673 , p. 195-217 , doi : 10.1246 / bcsj.78.195 .
- ↑ Takeshi Takeda, Rika Sasaki, Tooru Fujiwara: Carbonyl Olefination by Means of a Gem -Dichloride - Cp 2 Ti [P (OEt) 3] 2 system . In: The Journal of Organic Chemistry . tape 63 , no. October 21 , 1998, ISSN 0022-3263 , p. 7286-7288 , doi : 10.1021 / jo980724h .
- ↑ Bernhard Breit: Dithioacetals as an Entry to Titanium – Alkylidene Chemistry: A New and Efficient Carbonyl Olefination . In: Angewandte Chemie International Edition . tape 37 , no. 4 , 1998, ISSN 1521-3773 , pp. 453-456 , doi : 10.1002 / (SICI) 1521-3773 (19980302) 37: 43.0.CO; 2-M .
- ↑ Hisatoshi Uehara, Tohru Oishi, Masayuki Inoue, Mitsuru Shoji, Yoko Nagumo, Masashi Kosaka, Jean-Yves Le Brazidec, Masahiro Hirama: Convergent synthesis of the HIJKLM ring fragment of ciguatoxin CTX3C . In: Tetrahedron . tape 58 , no. 32 , August 2002, p. 6493-6512 , doi : 10.1016 / S0040-4020 (02) 00660-9 .
![{\ displaystyle {\ ce {Cp2Ti [P (OEt) 3] 2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8a227b6db464e6574a00966938d5c4607300064d)