Tebufenpyrad
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
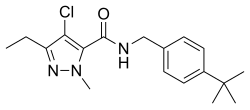
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tebufenpyrad | |||||||||||||||
| other names |
4-chloro- N - ((4- (1,1-dimethylethyl) phenyl) methyl) -3-ethyl-1-methyl-1 H -pyrazole-5-carboxamide |
|||||||||||||||
| Molecular formula | C 18 H 24 ClN 3 O | |||||||||||||||
| Brief description |
colorless solid with a faint odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 333.86 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.0214 g cm −3 |
|||||||||||||||
| Melting point |
64-66 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (2.8 mg l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tebufenpyrad is a chemical compound from the group of pyrazoles , which was introduced by Mitsubishi Chemical together with American Cyanamid (now BASF ) as a non-systemic acaricide .
Extraction and presentation
Tebufenpyrad can be obtained by reacting ethyl 4-chloro-3-ethyl-1-methyl-5-pyrazolecarboxylate with 4- tert- butylbenzylamine .
properties
Tebufenpyrad is a colorless solid with a faint odor that is practically insoluble in water.
use
Tebufenpyrad is used as an insecticide and acaricide. The effect is based on the inhibition of the mitochondrial electron transport complex 1 (METI).
Admission
Effective November 1, 2009, Tebufenpyrad was approved in the European Union for use as an insecticide and acaricide.
Tebufenpyrad had been approved in Germany since 1995, but, like in Austria, no longer.
In Switzerland, plant protection products (e.g. Masai, Zenar) with this active ingredient are permitted.
Web links
- EU: Review report for the active substance tebufenpyrad (PDF; 176 kB), December 1, 2008
Individual evidence
- ↑ a b c d e f EPA : Tebufenpyrad
- ↑ a b Entry on Tebufenpyrad in the Hazardous Substances Data Bank , accessed on August 7, 2012.
- ↑ Entry on Tebufenpyrad in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on tebufenpyrad (ISO); N- (4-tert-butylbenzyl) -4-chloro-3-ethyl-1-methyl-1Hpyrazole-5- carboxamide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the expand harmonized classification and labeling .
- ↑ Entry on Tebufenpyrad. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 517 (English, preview ).
- ^ Engineering Science Paper: Study on the Synthetic Process of Tebufenpyrad and Tolfenpyrad.
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . John Wiley & Sons, 2012, ISBN 978-3-527-32965-6 , pp. 1087 (English, limited preview in Google Book search).
- ↑ Commission Directive 2009/11 / EC of February 18, 2009 amending Council Directive 91/414 / EEC on the inclusion of bensulfuron, sodium 5-nitroguaiacolate, sodium o-nitrophenolate, sodium p-nitrophenolate and tebufenpyrad as Active ingredients
- ↑ Approval history of the BVL In: Reports on Plant Protection Products , 2009, p. 26.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Tebufenpyrad in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 6, 2019.



