Tetryl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetryl | ||||||||||||||||||
| other names |
|
||||||||||||||||||
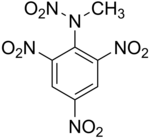
| Molecular formula | C 7 H 5 N 5 O 8 | ||||||||||||||||||
| Brief description |
yellowish monoclinic odorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 287.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.57 g cm −3 |
||||||||||||||||||
| Melting point |
129.4 ° C |
||||||||||||||||||
| boiling point |
187 ° C decomposes |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetryl ( N -Methyl- N -2,4,6-tetranitroaniline) is an energy-rich aromatic nitro compound . The substance from the group of nitramines used to be of great importance for the manufacture of detonators , but has now largely been replaced by more suitable compounds.
Extraction and presentation
Tetryl can be achieved by nitration of methyl aniline or dimethylaniline in the presence of sulfuric acid obtained. The preparation from dinitromethylaniline , which is accessible by condensation of methylamine and 2,4-dinitrochlorobenzene , is more suitable . The nitration of 2,4-dinitromethylaniline in dichloroethane with a mixture of nitric and sulfuric acid gives good tetryl yields.
properties
In its pure state, tetryl forms almost colorless crystals which quickly turn light yellow in light . The technical product is intense yellow. The compound melts at 129.5 ° C with a melting enthalpy of 22.93 kJ mol −1 . It reacts with aqueous bases , splitting off the methylnitramine group . Therefore, residual traces of acid in the crude product can only be removed by careful recrystallization .
Tetryl is insoluble in water, slightly soluble in ethanol and diethyl ether , more soluble in acetone or benzene . The melt has a flash point of 150 ° C. It is irritating to the respiratory tract and causes sensitization , contact dermatitis and yellowing of the skin.
Explosion parameters
The connection is particularly explosive when dry due to impact, friction, heat and other ignition sources and is subject to the Explosives Act . Important explosion indicators are:
Table with important explosion-relevant properties: Oxygen balance −47.4% Nitrogen content 24.39% Normal gas volume 939 l kg −1 Explosion heat 4251 kJ kg −1 (H 2 O (l))
4153 kJ kg −1 (H 2 O (g))Specific energy 1213 kJ kg −1 (123.7 mt / kg) Lead block bulge 41.0 cm 3 g −1 Detonation velocity 7850 m · s −1 Steel sleeve test Limit diameter 6 mm Sensitivity to impact 3 Nm Rubbing sensitivity 353 N pen load response Explosion temperature 180-190 ° C Deflagration point 185-195 ° C
The kinetics of thermal decomposition were investigated for the melt in the temperature range of 211 ° C and 2604 ° C. The decomposition process can be described with a first-order time law. The activation energy is 160.77 kJ · mol −1 , the Arrhenius factor 2.51 · 10 15 s −1 .
use
Tetryl was mainly used for detonators , explosive capsule fillings, intermittent charges and detonating cords . In the solidified melt with TNT, it was the first highly explosive explosive that was first used by Germany as a charge in artillery shells and torpedoes during the First World War . The substance has now been replaced by hexogen and pentrit , because, in addition to being slightly more explosive, they are also less toxic and cheaper to manufacture, although both are slightly more sensitive to impact.
Individual evidence
- ↑ a b c Entry on Tetryl. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d e f Entry on N-methyl-2,4,6-N-tetranitroaniline in the GESTIS substance database of the IFA , accessed on February 21, 2017(JavaScript required) .
- ↑ Entry on N-methyl-N, 2,4,6-tetranitroaniline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ lgcstandards: U-NAIM-833E-1 (PDF; 159 kB)
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 479-45-8 or Tetryl ), accessed on November 2, 2015.
- ↑ a b c d e f g h i j k l J. Köhler, R. Meyer, A. Homburg: Explosivstoffe. 10., completely revised. Edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 , p. 287.
- ^ G. Krien, HH Licht, J. Zierath: Thermochemical investigation of nitramines. In: Thermochim. Acta . 6, 1973, pp. 465-472.
- ↑ a b c d L. Roth, U. Weller: Hazardous chemical reactions , entry for Tetryl, status 56. Supplementary delivery 11/2008, ecomed Verlag Landsberg / Lech, ISBN 978-3609195872 .
- ↑ Explosives Act, Appendix I, List of Explosive Substances ( BGBl. 1975 I p. 853 ), to which the law is to be applied in full.
- ^ A b A. JB Robertson: The thermal decomposition of explosives. Part I. Ethylenedinitramine and tetryl. In: Trans. Faraday Soc. 44, 1948, pp. 677-682, doi: 10.1039 / TF9484400677 .
literature
- Kast: Journal. Ges. Sch. Spr. 445 (1912)
- CJ Bain: Army Ordnance. 6, 435 (1926)
- G. Desseigne: Mém. Poudres. 28, 1938, pp. 156-170.
- RC Elderfield: Study of the British Continuous Tetryl Process. Rept. 661, OSRD (1942)
- J. Issoire, G. Burlet: Mém. Poudres. 39, 1957, pp. 65-95, 40, 1958, pp. 47-76.
- Brit. Pat. 919,717 (Feb. 27, 1963; US Appl. - Aug. 17, 1959, 9pp, US Rubber Co.)
- T. Urbanski: Chemistry and Technology of Explosives. Vol. 3, Pergamon Press, New York 1967, p. 40.
- US Military Spec. T-338C, USGPO, (1971)
- A. Stettbacher: The guns and explosives. 2nd Edition. Leipzig 1933.


