Thidiazuron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
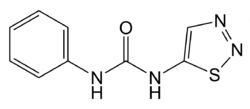
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Thidiazuron | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 8 N 4 OS | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 220.25 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.51 g cm −3 |
|||||||||||||||
| Melting point |
211.5 ° C |
|||||||||||||||
| solubility |
practically insoluble in water (20 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thidiazuron is a chemical compound from the group of phenylureas .
Extraction and presentation
Thidiazuron can be obtained by reacting 3-amino-1,2-thiadiazole and phenyl isocyanate .
use
Thidiazuron has been used as a growth regulator with a cytokinin- like effect and as a defoliant in cotton cultivation since the 1980s .
Admission
The use of the active ingredient thidiazuron in plant protection products is not permitted in the European Union or Switzerland. However, it is known that the active ingredient manufactured by Bayer is sold on the Brazilian and South African markets.
Individual evidence
- ↑ a b c d e f Entry on thidiazuron in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on March 24, 2014.
- ↑ a b Data sheet Thidiazuron from Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 258 ( limited preview in Google Book search).
- ↑ Patent US4294605 : Agents for the defoliation of plants. Applied on November 21, 1979 , published October 13, 1981 , Applicant: Schering. And patent DE2214623.
- ↑ MC Mok, DWS Mok, DJ Armstrong, K. Shudo, Y. Isogai, T. Okamoto: Cytokinin activity of N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (thidiazuron) . In: Phytochemistry . tape 21 , no. January 7 , 1982, pp. 1509-1511 , doi : 10.1016 / S0031-9422 (82) 85007-3 ( PDF ).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on thidiazuron in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.
- ↑ Decision of the Commission of April 4, 2008 (PDF) on the non-inclusion of azocyclotine, cyhexatin and thidiazuron in Annex I of Council Directive 91/414 / EEC.
- Jump up ↑ Benjamin Luig, Fran Paula de Castro and Alan Tygel (both Campanha Permanente Contra os Agrotóxicos e Pela Vida), Lena Luig (INKOTA network), Simphiwe Dada (Khanyisa), Sarah Schneider (MISEREOR) and Jan Urhahn (Rosa-Luxemburg- Foundation): Dangerous pesticides. (PDF; 2.4 MB) from Bayer and BASF - a global business with double standards. Rosa Luxemburg Foundation , INKOTA network , Episcopal Aid Organization Misereor u. a., April 2020, accessed on April 25, 2020 .

