Thymidylate synthase
| Thymidylate synthase | ||
|---|---|---|
|
||
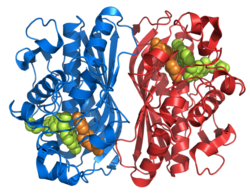
| Ribbon model of the thymidylate synthase dimer, complexed with the inhibitor raltitrexed (calottes) according to PDB 1HVY | ||
|
Existing structural data: 1hvy, 1hw3, 1hw4, 1hzw, 1i00, 1ju6, 1juj, 1ypv, 2onb |
||
| Properties of human protein | ||
| Mass / length primary structure | 313 aa; 35.7 kDa | |
| Secondary to quaternary structure | Homodimer | |
| Identifier | ||
| Gene names | TYMS ; TS; HsT422; MGC88736; TMS; TSase | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 2.1.1.45 , transferase | |
| Response type | Reductive methylation | |
| Substrate | dUMP + 5,10-methylene tetrahydrofolate | |
| Products | dTMP + dihydrofolate | |
| Occurrence | ||
| Parent taxon | Creature | |
| Orthologue | ||
| human | mouse | |
| Entrez | 7298 | 22171 |
| Ensemble | ENSG00000176890 | ENSMUSG00000025747 |
| UniProt | P04818 | Q544L2 |
| Refseq (mRNA) | NM_001071 | NM_021288 |
| Refseq (protein) | NP_001062 | NP_067263 |
| Gene locus | Chr 18: 0.65 - 0.66 Mb | Chr 5: 30.39 - 30.4 Mb |
| PubMed search | 7298 |
22171
|
Thymidylate synthase is an enzyme involved in the biosynthesis of dTMP, ( d for deoxynucleotides; TMP for thymidine monophosphate ) which methylates uracil in the form of uridine monophosphate. The presence of sufficient amounts of dTMP is necessary for DNA repair and its replication . The enzyme is therefore the target for cytostatic drugs such as 5-fluorouracil , tegafur , capecitabine , methotrexate , pemetrexed and raltitrexed , which inhibit it. Resistance to these drugs occurs in people who produce an excess of the enzyme. TYMS competes with the enzyme MTHFR , which converts homocysteine to methionine , for the cofactor methylene tetrahydrofolate .
synthesis
The TYMS for encoding gene is present in all eukaryotes to find. In humans, it extends over 15 kilobases in 6 exons on chromosome 18 . The protein is 313 amino acids long and 35.7 kDa in weight.
Catalyzed reaction
The synthesis of dTMP (1b) from dUMP (1a) is catalyzed by thymidylate synthase (A). The methyl group comes from N5, N10-methylene tetrahydrofolate (2), which is converted to 7,8-dihydrofolate (3). The radical R 1 is 1- (2-deoxy-β- D -ribofuranosyl) -, R 2 is -benzoylglutamic acid.
Individual evidence
- ↑ Seymour S. Cohen, Joel G. Flaks, Hazel D. Barner, Marilyn R. Loeb, Janet Lichtenstein: The Mode of Action of 5-Fluorouracil and Its Derivatives . In: Proceedings of the National Academy of Sciences . tape 44 , no. 10 , October 15, 1958, ISSN 0027-8424 , p. 1004-1012 , doi : 10.1073 / pnas.44.10.1004 , PMID 16590300 ( pnas.org [accessed June 2, 2018]).
- ↑ Thymidylate Synthase. In: Online Mendelian Inheritance in Man . (English).
- ↑ Ensembl entry
