Tegafur
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -form (left) and ( S ) -form (right) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tegafur | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 9 FN 2 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 200.17 g · mol -1 | |||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Tegafur ( INN ) is a drug that is used as a cytostatic agent in the chemotherapy of metastatic cancer, especially stomach, colon and breast cancer. The drug belongs to the group of nucleoside - analogues . In the liver , it is in 5-fluorouracil converted (5-FU), and thus relates to a prodrug , which in contrast to 5-FU orally may be administered.
Tegafur is also given in combination with uracil , with which the compound forms a 1: 1 adduct. The uracil inhibits the 5-FU-degrading enzyme dihydropyrimidine dehydrogenase (DPD).
Stereoisomerism
Tegafur contains a stereogenic center and is therefore chiral , so there are two enantiomers , ( R ) -5-fluoro-1- (tetrahydro-2-furyl) -uracil and the mirror image ( S ) -5-fluoro-1- (tetrahydro -2-furyl) uracil. The drug Tegafur is used as a racemate [1: 1 mixture of ( R ) -5-fluoro-1- (tetrahydro-2-furyl) -uracil and ( S ) -5-fluoro-1- (tetrahydro-2-furyl) -uracil], although the different pharmacodynamics of drug enantiomers - apart from rare exceptions - are known.
synthesis
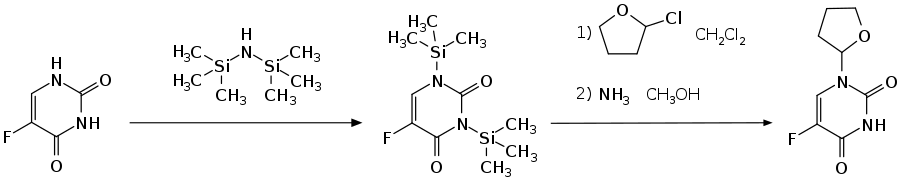
The synthesis of Tegafur starts from 5-fluorouracil , which is silylated on the amide functions in the first step using hexamethyldisilazane . The tetrahydrofuranyl group is introduced by reaction with 2-chlorotetrahydrofuran . The target molecule is then obtained by splitting off the trimethylsilyl group using a methanolic ammonia solution .
In a second synthesis variant, 5-fluorouracil is reacted directly with 2,3-dihydrofuran in the presence of calcium chloride and pyridine to give tegafur.
Both synthesis sequences result in the racemate .
properties
Racemic tegafur occurs in four polymorphic forms. The α, β and δ forms can be obtained by solvent crystallization. The γ form is formed after enantiotropic transformations from the α or β form at 162 ° C and 120 ° C and then melts at 175 ° C. The δ form shows a melting point at 165 ° C. The crystal structures of the α and β forms have been examined by means of X-ray single crystal examinations. The α – form crystallizes in a triclinic crystal lattice with the space group P 1 (space group no. 1) . A monoclinic crystal lattice with the space group P 2 1 / n (no. 14, position 2) was determined for the β form . Both forms form dimers linked by hydrogen bonds in the crystal lattice. The difference is that in the α form a link is made via the NH group and the urea-analogous carbonyl structure and in the β form this is via the NH group and the acid amide-analogous carbonyl structure.
Individual evidence
- ↑ a b c d e f g T. Uchida, E. Yonemochi, T. Oguchi, K. Terada, K. Yamamoto, Y. Nakai: Polymorphism of Tegafur: Physico-chemical Properties of Four Polymorphs. In: Chem. Pharm. Bull. 41, 1993, pp. 1632-1635.
- ↑ a b Tegafur data sheet at Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ a b c d e f g A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag Stuttgart 2000, ISBN 1-58890-031-2 .
- ↑ NI Karev, NG Blokhina, EK Vozny, MP Pershin: Experience with ftorafur treatment in breast cancer. In: neoplasm. 19, 1972, pp. 347-350.
- ^ EJ Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. In: European Journal of Clinical Pharmacology . 26, 1984, pp. 663-668, doi: 10.1007 / BF00541922 .
- ↑ E. Lukevits, A. Zablotskaya: Synthesis of Ftorafur (review). In: Chemistry of Heterocyclic Compounds. 27, 1992, pp. 1271-1299, doi: 10.1007 / BF00515572 .
- ^ Y. Nakai, K. Yamamoto, K. Terada, T. Uchida, N. Shimizu, S. Nishigaki: The Crystal Structure of Ftorafur. In: Chem. Pharm. Bull. 30, 1982, pp. 2629-2632, doi: 10.1248 / cpb.30.2629 , (pdf)
- ^ F. Needham, J. Faber, TG Fawcett: X-ray powder diffraction analysis of tegafur. In: Powder Diffraction. 21, 2006, pp. 245-247, doi: 10.1154 / 1.2210952 .
- ^ A b Y. Nakai, K. Yamamoto, K. Terada, T. Uchida, K. Yamaguchi, N. Shimizu: The crystal structure of Tegafur (β – form): Comparison with α – form. In: Chem. Pharm. Bull. 34, 1986, pp. 1242-1248, doi: 10.1248 / cpb.34.1242 , (pdf)
literature
- HJ Roth, H. Fenner: Medicines . Thieme, Stuttgart / New York 1988, ISBN 3-13-673501-3 , pp. 198-199.
Trade names
- D, CH UFT ® ( Merck KGaA ) for the tegafur-uracil adduct
- I Citofur (Lusofarmaco)
- J Coparogin (Nippon Chemiphar)
- J Flulaid (Takeda)