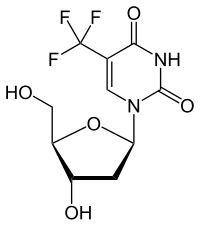
Trifluridine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trifluridine | ||||||||||||||||||
| other names |
2'-deoxy-5- (trifluoromethyl) uridine |
||||||||||||||||||
| Molecular formula | C 10 H 11 F 3 N 2 O 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 296.2 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
190-193 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Trifluridine (as a combination preparation with Tipiracil Lonsurf ® ; manufacturer Servier ) is a drug from the group of nucleoside analogs .
The compound was patented by the U.S. Department of Health in 1970 and is used as an antiviral drug .
The combination of trifluridine and tipiracil (formerly TAS-102) in a molar ratio of 1: 0.5 is used for the oral treatment of metastatic colorectal cancer in adult patients after various previous therapies . It has been approved in the EU since April 2016.
application areas
The combination of trifluridine and tipiracil has been approved in the EU since April 2016 for the treatment of adult patients with metastatic colorectal cancer who have already been treated with available therapies or who are not suitable for them. These therapies include fluoropyrimidine, oxaliplatin and irinotecan based chemotherapy, anti- VEGF and anti- EGF receptor substances. Approval was based on the RECOURSE study, a prospectively randomized, placebo-controlled phase III study with 800 patients. The randomization was carried out in the ratio verum: placebo 2: 1, in each case with the best possible support (BSC = Best Supportive Care). The study was able to show a statistically significant advantage in the primary endpoint, overall survival (OS). Under this treatment, patients survived an average of 7.1 months in the verum arm and 5.3 months in the placebo arm (p = 0.001). The mean time to disease progression (PFS = Progression Free Survival) was two months in the combination trifluridine / tipiracil and 1.7 months in the placebo arm. A crossover was not permitted until the final analysis of the primary endpoint (OS). Additional clinical study endpoints were overall tumor response rate (ORR) and disease control rate (DCR).
Mode of action and properties
The combination preparation consists of the nucleosidic thymidine analogue trifluridine (TF; it should actually be called trifluorothymine) and the thymidine phosphorylase inhibitor tipiracil (TPI). The chemical names according to IUPAC are:
- Trifluridine: 1 - [(2 R , 4 S , 5 R ) -4-hydroxy-5- (hydroxymethyl) tetrahydrofuran-2-yl] -5- (trifluoromethyl) pyrimidine-2,4 (1 H , 3 H ) - dion.
- Tipiracil hydrochloride: 5-chloro-6 - [(2-iminopyrrolidin-1-yl) methyl] pyrimidin-2,4 (1 H , 3 H ) -dione as the hydrochloride salt.
The molar quantity ratio of trifluridine / tipiracil is 1: 0.5 (exact mass ratio: 1: 0.471). TF is phosphorylated intracellularly to monophosphate (TF-MP) by the enzyme thymidine kinase and subsequently to di- (TF-DP) and triphosphate (TF-TP) by the enzyme thymidylate kinase. TF-TP is built into the DNS as a wrong component. Long-lasting DNA damage and DNA strand breaks result from this incorrect incorporation. The precise mechanism for this is still unclear. TF-MP in turn binds covalently to tyrosine-146 in the active center of the enzyme thymidylate synthase (TS, also thymidilate synthase ) and inhibits its activity. TS is responsible for the conversion of uracil nucleotides into thymidine nucleotides and is therefore of vital importance for DNA synthesis by maintaining a sufficient amount of thymidine. Thymidine is only found in DNA but not in RNA. Uridine can be found there.
Tipiracil
The TS inhibition can only be maintained if, as with a continuous infusion, TF would be replenished. Otherwise the TS recovers quickly and the cytotoxic effect is over. TF is quickly inactivated by the enzyme thymidine phosphorylase (TP) during absorption from the intestine, liver, spleen and also intracellularly.
Analogous to thymidine, trifluridine is broken down by TP to trifluorothymine and 2-deoxyribose-1-phosphate. This reaction can be effectively prevented by adding the TP inhibitor Tipiracil. This makes it possible to simulate the intracellular kinetics of TF like a continuous infusion. However, it is necessary to take trifluridine / tipiracil twice a day (see below).
Another effective quality of Tipiracil is its antiangiogenic properties. In the treatment of colorectal cancer and other tumors , drugs are used against the tumor-related formation of new blood vessels ( angiogenesis ). As early as the 1990s it was discovered that TP has proangiogenic properties or that it is identical to the endothelial cell growth factor PD-ECGF (PD-ECGF = platelet-derived endothelial cell growth factor), which is derived from platelets. Later research has confirmed this. On the one hand, these properties could contribute to the fact that trifluridine / tipiracil is still effective in heavily pretreated patients and the development of drug resistance is delayed or prevented. Whether the dose of tipiracil contained in the combination is sufficient for significant antiangiogenesis in colorectal cancer patients cannot necessarily be deduced.
Difference to fluorouracil (5-FU)
5-FU is an antimetabolite from the group of pyrimidine analogues and is similar to uracil (see fig.). 5-FU is also not active, but has to be metabolized into the corresponding ribo- and deoxyribonucleotides (5-fluorouridine, 5-fluorodeoxyuridine). In addition to being incorporated into the RNA, it is converted to 5-fluoro-2'-deoxyuridine-5'-monophosphate (5-FdUMP). This binds to the thymidilate synthetase and inhibits it. This blocks the conversion of deoxyuridine monophosphate to deoxythymidine monophosphate and inhibits DNA synthesis . A "thymineless state" arises - the cell dies because not enough thymine / thymidine is available for DNA synthesis. The DNA-directed cytotoxicity can be increased further by exogenously increasing the intracellular level of reduced folates . By administering calcium folinate (N-5-formyl tetrahydrofolate calcium salt) the binding of 5-FdUMP to the thymidilate synthetase is strengthened by the formation of a ternary non-covalent complex consisting of calcium folinate-5-FdUMP thymidilate synthetase. This increases the duration of the 5-fluorouracil-mediated DNA synthesis inhibition. In this combination, the cytotoxicity is increased. The anti-tumor effect of TF is mainly caused by the incorrect incorporation into DNA than by the TS inhibition.
In the mouse model, TF is incorporated into the DNA to a greater extent than other anti-tumor nucleosides. Surprisingly, orally applied TF is more effectively incorporated into the DNA than continuously infused trifluridine with simultaneously increased anti-tumor activity and better tolerability. Combined as TAS-102, TF gradually accumulated in tumor DNA regardless of TPI. Compared to 5-FU derivatives, TF / TPI significantly delayed tumor growth and prolonged survival in mouse experiments.
5-FU is similar to uracil. By substituting fluorine in position 5, the TS cannot synthesize thymine. With trifluridine, the deoxynucleoside, which is similar to thymidine, is already available. Here the methyl group has been replaced by trifluorination, which inhibits the enzyme by binding to the catalytic center. In preclinical studies, trifluridine / tipiracil were tested against both 5-fluorouracil (5-FU) -sensitive and resistant colorectal tumor cell lines. The cytotoxic activity of trifluridine / tipiracil in different human tumor xenografts correlated strongly with the amount of trifluridine incorporated into the DNA, which suggests that the DNA incorporation is the primary mechanism of action (see above).
Dosage forms
The finished medicinal product of trifluridine / tipiracil is available in the dose strengths 15 mg trifluridine / 6.14 mg tipiracil and 20 mg trifluridine / 8.19 mg tipiracil.
Dosages and dose modifications
Trifluridine / tipiracil is dosed based on the patient's body surface [mg / m²]. The basic dose is 35 mg / m² twice a day for days 1 - 5 in the first two weeks. This is followed by a 16-day break in a 28-day cycle (see table). The film-coated tablets should be taken 1 hour after breakfast and 1 hour after the evening meal.
| Overview of trifluoridine / tipiracil intake within a 28-day cycle | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dose | week 1 | Week 2 | Week 3 | Week 4 | 28 d = 1 cycle | ||||||||||||||
| Trifluoridine / tipiracil | 2 x 35 mg / m 2 | Days | 1 | 2 | 3 | 4th | 5 | x | x | 1 | 2 | 3 | 4th | 5 | x | x | Break | Break | Break: 16 d |
The respective dose must be rounded to the nearest 5 mg step. A total dose of 80 mg should not be exceeded (corresponds to a person with a calculated body surface area of 2.28 m²). Dose reductions can be made depending on individual tolerance and safety. However, the dosage should not fall below 20 mg / m².
Side effects
The combination can cause severe myelosuppression with anemia , neutropenia, and thrombocytopenia . This can result in severe infections. Therefore, a whole blood count must be performed before starting therapy and at least before each treatment cycle. Further side effects regardless of severity and frequency are, according to the European specialist information: gastrointestinal complaints such as nausea, diarrhea, vomiting, abdominal pain, stomatitis, asthenia / fatigue, pyrexia, decreased appetite, taste disturbance, hair loss.
Contraindications
Currently none set.
Contraindications
Hypersensitivity to trifluridine and / or tipiracil.
Pharmacokinetics
Absorption / bioavailability
After oral administration of trifluridine / tipiracil, at least 57% / 27% of the administered amount is absorbed. Time to maximum plasma concentration (tmax) of trifluridine / tipiracil approx. 2 or 3 hours. Excretion in the faeces: trifluridine / tipiracil 3% / 50% plasma protein binding: trifluridine / tipiracil 96%, 7> 8% Apparent volume of distribution (Vd / F) of trifluridine / tipiracil 21 l / 333 l
Biotransformation and Elimination
Trifluridine is mainly eliminated by conversion into its inactive main metabolite 5- [trifluoromethyl] uracil (TFMU) by means of TP in the urine. Less common metabolites are 5-carboxyuracil and 5-carboxy-2'-deoxyuridine and are also found in small amounts or traces in urine.
A population pharmacokinetic analysis revealed no clinically relevant effects of age, gender, race on the pharmacokinetics of trifluridine or tipiracil.
Interactions
In vitro studies showed that neither trifluridine nor tipiracil nor the inactive main metabolite TMFU inhibit the activity of human cytochrome P450 (CYP) isozymes. An inducing effect of tipiracil on human CYP isoenzymes cannot be excluded. In vitro , trifluridine is a substrate for the nucleoside transporters CNT1, ENT1 and ENT2. Tipiracil is a substrate for the organic cation transporter polypeptide 2 (OCT2) and the SLC transporter MATE1 (Multidrug And Toxin Extrusion 1).
When using drugs that belong to the human thymidine kinase substrates, e.g. B. certain antivirals , these drugs, when used at the same time as trifluridine / tipiracil, can compete with trifluridine for activation by the thymidine kinase and exhibit reduced effectiveness. If simultaneous antiviral therapy is unavoidable, thymidine kinase-independent active substances should be used.
Historical
Trifluridine and 5-FU were introduced into therapy at the same time in the 1960s. Until the development of the protracted infusions, 5-FU was used extensively, but with moderate success. Trifluridine alone (!) Would have because of the above. Kinetics must be given iv every 3 hours (including at night) until a daily dose of 2.5 mg / kg is reached. In principle, the tests with trifluridine at the time showed that the substance had anti-tumor activity. However, the development as a cytostatic had to be discontinued because the toxicity was too great at the required doses. Because of its existing antiviral activity against herpes simplex , trifluridine was marketed in various countries in the form of eye drops. As an antiviral agent, it is / was used to treat keratitis caused by herpes simplex viruses.
Web links
Individual evidence
- ↑ a b c Data sheet Trifluorothymidine, ≥99% (HPLC) from Sigma-Aldrich , accessed on June 16, 2016 ( PDF ).
- ↑ Entry on trifluridine. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2016.
- ↑ a b EMA. Lonsurf . 2016.
- ↑ a b EMA. Lonsurf - Summary of Opinion . 2016.
- ↑ a b c d Robert J. Mayer, Eric Van Cutsem, Alfredo Falcone, Takayuki Yoshino, Rocio Garcia-Carbonero, Nobuyuki Mizunuma, Kentaro Yamazaki, Yasuhiro Shimada, Josep Tabernero, Yoshito Komatsu, Alberto Sobrero, Eveline Boucher, Marc Peeters, Ben Tran, Heinz-Josef Lenz, Alberto Zaniboni, Howard Hochster, James M. Cleary, Hans Prenen, Fabio Benedetti, Hirokazu Mizuguchi, Lukas Makris, Masanobu Ito, Atsushi Ohtsu: Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer . In: New England Journal of Medicine . tape 372 , no. May 20 , 2015, p. 1909-1919 , doi : 10.1056 / NEJMoa1414325 , PMID 25970050 .
- ↑ Irene V. Bijnsdorp, Godefridus J. Peters, Olaf H. Temmink, Masakazu Fukushima, Frank A. Kruyt: Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells . In: International Journal of Cancer . tape 126 , no. 10 , May 2010, p. 2457-2468 , doi : 10.1002 / ijc.24943 .
- ↑ a b c d e f Olaf H. Temmink, Tomohiro Emura, Michiel De Bruin, Masakazu Fukushima, Godefridus J. Peters: Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies . In: Cancer Science . tape 98 , no. 6 , June 2007, p. 779-789 , doi : 10.1111 / j.1349-7006.2007.00477.x .
- ↑ Kensuke Usuki, Jan Saras, Johannes Waltenberger, Kohei Miyazono, Glenn Pierce, Arlen Thomason, Carl-Henrik Heldin: Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity . In: Biochemical and Biophysical Research Communications . tape 184 , no. 3 , May 1992, pp. 1311-1316 , doi : 10.1016 / S0006-291X (05) 80025-7 .
- ↑ a b Godefridus Peter J. email, Irene V. Bijnsdorp: TAS-102: more than at antimetabolite . In: The Lancet Oncology . tape 13 , no. December 12 , 2012, p. e518-e519 , doi : 10.1016 / S1470-2045 (12) 70426-6 .
- ↑ Barth, J., Mechanisms of action of cytostatics, in Zytostatika in der Apotheke, J. Barth, Editor. 2015, pp. 37–40.
- ↑ a b Nozomu Tanaka, Kazuki Sakamoto, Hiroyuki Okabe, Akio Fujioka, Keisuke Yamamura, Fumio Nakagawa, Hideki Nagase, Tatsushi Yokogawa, Kei Oguchi, Keiji Ishida, Akiko Osada, Hiromi Kazuno, Yukari Yamada, Kenichi Matsuo: Repeated oral of dosing , Kenichi Matsuo -102 confers high trifluridine incorporation into DNA and sustained antitumor activity in mouse models . In: Oncology Reports . tape 32 , no. 6 , 2014, p. 2319–2326 , doi : 10.3892 / or.2014.3487 .
- ↑ Charles. Heidelberger, David. Parsons, David C. Remy: Syntheses of 5-Trifluoromethyluracil and 5-Trifluoromethyl-2 ″ -deoxyuridines . In: Journal of the American Chemical Society . tape 84 , no. 18 , 1962, pp. 3597-3598 , doi : 10.1021 / ja00877a046 .
- ↑ Paulo M. Hoff, Jim Cassidy, Hans-Joachim Schmoll: The Evolution of Fluoropyrimidine Therapy: From Intravenous to Oral . In: The Oncologist . tape 6 , Supplement 4, 2001, pp. 3-11 , doi : 10.1634 / theoncologist.6-suppl_4-3 .
- ↑ David S. Hong, James L. Abbruzzese, Karla Bogaard, Yvonne Lassere, Masakazu Fukushima, Akira Mita, Keizo Kuwata, Paulo M. Hoff: Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors . In: Cancer . tape 107 , no. 6 , 2006, p. 1383-1390 , doi : 10.1002 / cncr.22125 .

