Trimedlure
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structure of 4- (above) and 5-chloro-2-methylcyclohexanecarboxylic acid butyl ester (below), in each case without representation of the stereochemistry, see composition | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimedlure | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 21 ClO 2 | |||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 230.6 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| Melting point |
20 ° C |
|||||||||||||||
| boiling point |
107-113 ° C (0.6 torr) |
|||||||||||||||
| solubility |
hardly soluble in water (1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trimedlure is the name for a mixture of several isomeric chemical compounds from the group of carboxylic acid esters . Trimedlure belongs to the insect pheromones and is the most effective synthetic sexual attractant of the Mediterranean fruit fly . The (1 S , 2 S , 4 R ) isomer has the highest potency. The iodine derivative is known under the name Ceralure .
Presentation and extraction
The production of Trimedlure starts from the trans -6-methyl-3-cyclohexenecarboxylic acid , to which hydrogen chloride is added in the first step . This step is not stereospecific, so that the 4-chloro and 5-chloro isomers of the trans -2-methylcyclocarboxylic acid are formed. In the second step, an esterification takes place by reaction with isobutene . The synthesis sequence produces a mixture of four of the theoretically possible eight racemic stereoisomers, which can be separated by means of preparative chromatography. An enantiomerically pure representation of the most biologically active ( 1S , 2 S , 4 R ) and (1 S , 2 S , 5 R ) isomers is possible by using the corresponding enantiomerically pure starting compounds of trans -6-methyl-3-cyclohexenecarboxylic acid.
Composition and properties
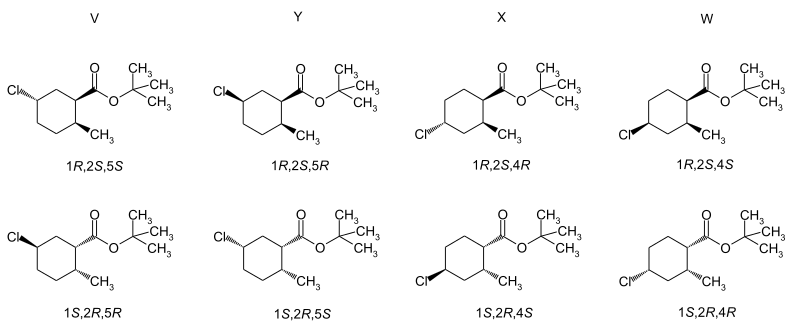
Trimedlure is a mixture of different isomers . Because of the different chlorine substitution resulting from the synthesis, there is a mixture of two structural isomers . Each of the molecules contains three stereocenters , so that four cis-trans isomers can be formulated, each of which in turn forms a pair of enantiomers . So a total of 16 different isomers are possible. Since the synthesis starts from the trans -6-methyl-3-cyclohexenecarboxylic acid, the resulting trans isomers are essentially contained in the commercial product . In the literature, the four pairs of enantiomers are named A, B 1 , B 2 and C. The composition is about 35% A, 5% B 1 , 10% B 2 and 50% C. The most biologically active isomer is the (1 S , 2 S , 4 R ) -enantiomer of the C-enantiomer pair. In the case of the structurally isomeric A enantiomer pair, the (1 S , 2 S , 5 R ) enantiomer is also much more biologically active.
The cis isomers are contained in the commercial product with about 5%. The literature uses the designation of the enantiomer pairs with V, W, X and Y. Chromatographic separation and isolation is possible.
Little information is available about the properties of the Trimedlure isomers. The racemates of the 4-chloro derivative exist as low-melting, crystalline solids, while those of the 5-chloro derivative form viscous oils.
| isomer | Type | CAS number | Melting point | Refractive index | Rotation value |
|---|---|---|---|---|---|
| A. | Racemate | 92284-32-7 | 1.4579 | ||
| A. | (1 R , 2 R , 5 S ) enantiomer | 92344-63-3 | −4.7 ° | ||
| A. | (1 S , 2 S , 5 R ) enantiomer | 92344-64-4 | + 6.7 ° | ||
| B 1 | Racemate | 92314-24-4 | 1.4576 | ||
| B 1 | (1 R , 2 R , 5 R ) enantiomer | 92344-65-5 | + 25.7 ° | ||
| B 1 | (1 S , 2 S , 5 S ) enantiomer | ||||
| B 2 | Racemate | 92284-33-8 | 71-72 ° C | ||
| B 2 | 1 R , 2 R , 4 R enantiomer | 92344-66-6 | −19.2 ° | ||
| B 2 | 1 S , 2 S , 4 S enantiomer | 92344-67-7 | + 27.9 ° | ||
| C. | Racemate | 92284-34-9 | 57-58 ° C | ||
| C. | (1 R , 2 R , 4 S ) enantiomer | 92344-68-8 | −20.4 ° | ||
| C. | (1 S , 2 S , 4 R ) enantiomer | 92344-69-9 | + 19.6 ° |
Admission
The use of the active ingredient trimedlure in plant protection products is not permitted in the European Union or in Switzerland.
Individual evidence
- ↑ a b c Robert Krieger (Ed.): Hayes' Handbook of Pesticide Toxicology . Elsevier, 1999, ISBN 978-0-12-374367-1 , pp. 172 (English, limited preview in Google Book Search).
- ^ Interchem Technologies
- ↑ a b c Beroza, M .; Green, N .; Gertler, SI: New Attractants for the Mediterranean Fruit Fly in J. Acricul. Food Chem. 9 (1961) 361-365, doi: 10.1021 / jf60117a007 .
- ↑ Entry on Trimedlure in the BioPesticide Properties DataBase (BPDB) of the University of Hertfordshire , accessed on November 30, 2013.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1,1-dimethylethyl 4 (or 5) -chloro-2-methylcyclohexanecarboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 28, 2017, is reproduced from a self-classification by the distributor .
- ↑ a b M. Beroza et al .: Acute toxicity studies with insect attractants . Toxicol Appl Pharmacol. 1975 (3): 421-429. doi : 10.1016 / 0041-008X (75) 90264-1
- ↑ a b c d e f T. M. McGovern, M. Beroza: Structure of the Four Isomers of the Insect Attractant Trimedlure . In: J. Org. Chem. Band 31 , 1966, pp. 1472–1477 , doi : 10.1021 / jo01343a036 (English).
- ↑ a b P. E. Sonnet, TP McGovern, RT Conningham: Enantiomers of the biologically active components of the insect attractant trimedlure . In: J. Org. Chem. Band 49 , 1984, pp. 4639–4643 , doi : 10.1021 / jo00198a012 (English).
- ↑ a b Doolittle, RE; Cunningham, RT; McGovern, TP; Sonnet, PE: Trimedlure enantiomers: differences in attraction for mediterranean fruit fly, Ceratitis capitata (Wied.) (Diptera: Tephritidae) in J. Chem. Ecol. 17 (1991) 475-484, doi: 10.1007 / BF00994346 .
- ↑ a b Warthen, JD; McGovern, TP: Purification of cis-trimedlure isomers by high-performance liquid chromatography in Chromatographia 21 (1986) 651-654, doi: 10.1007 / BF02311922 .
- ↑ a b Warthen, JD; McGovern, TP: Semi-preparative high-performance liquid chromatographic separation of trimed acid isomers in Chromatographia 25 (1988) 811-814, doi: 10.1007 / BF02262090 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Trimedlure in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.
- ↑ Decision of the Commission of January 30, 2004 on the non-inclusion of certain active substances in Annex I of Council Directive 91/414 / EEC and the revocation of the authorizations for plant protection products with these active substances (2004/129 / EC)