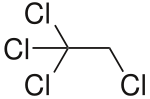
1,1,1,2-tetrachloroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1,1,2-tetrachloroethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 Cl 4 | |||||||||||||||
| Brief description |
volatile, colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 167.85 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.6 g cm −3 |
|||||||||||||||
| Melting point |
−70.2 ° C |
|||||||||||||||
| boiling point |
138 ° C |
|||||||||||||||
| Vapor pressure |
18.6 mbar (25 ° C) |
|||||||||||||||
| solubility |
heavy in water (1.07 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.481 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1,1,1,2-Tetrachloroethane is a chemical compound from the group of aliphatic saturated halogenated hydrocarbons and organic chlorine compounds . It is isomeric to 1,1,2,2-tetrachloroethane .
Extraction and presentation
1,1,1,2-Tetrachloroethane can be obtained by a two-stage addition reaction of ethyne with chlorine (via dichloroethene ), whereby 1,1,2,2-tetrachloroethane is mainly formed.
It can be obtained directly by chlorinating 1,1,2-trichloroethane .
It also occurs as a contaminant in the manufacture of some common chlorinated hydrocarbons.
properties
1,1,1,2-Tetrachloroethane is a flammable, volatile, colorless liquid that is not very soluble in water. It pyrolyzes at 550–650 ° C , producing chlorine and hydrogen chloride , among other things .
use
It is used as a solvent and for the production of glazes and varnishes, and as an intermediate product for the production of trichloroethene and tetrachloroethene . In the USA it has also been detected in low concentrations in the air and in drinking water.
safety instructions
The IARC classified 1,1,1,2-tetrachloroethane as possibly carcinogenic in 2014.
Individual evidence
- ↑ a b c d e f g h i j Entry on 1,1,1,2-tetrachloroethane in the GESTIS substance database of the IFA , accessed on February 10, 2017(JavaScript required) .
- ↑ Data sheet 1,1,1,2-tetrachloroethane from Sigma-Aldrich , accessed on March 5, 2011 ( PDF ).
- ^ Lawrance Waddams: The Petroleum chemicals Industry , p. 175.
- ^ A b International Agency for Research on Cancer : 1,1,1,2-Tetrachloroethane , 1987.
- ↑ IARC Monograph 106 - 1,1,1,2-Tetrachloroethane, 2014
literature
- EN Culubret, M. Luz, R. Amils, JL Sanz: Biodegradation of 1,1,1,2-tetrachloroethane under methanogenic conditions . In: Water Science and Technology : A Journal of the International Association on Water Pollution Research . tape 44 , no. 4 , 2001, p. 117-122 , PMID 11575074 .





