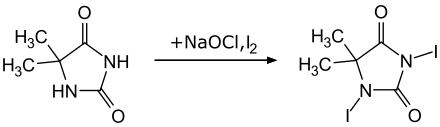
1,3-diiodo-5,5-dimethylhydantoin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
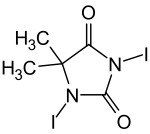
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,3-diiodo-5,5-dimethylhydantoin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 6 I 2 N 2 O 2 | ||||||||||||||||||
| Brief description |
pale yellow to light brown powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 379.92 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
192-196 ° C |
||||||||||||||||||
| solubility |
soluble in acetone , slightly soluble in dichloromethane |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
1,3-Diiodo-5,5-dimethylhydantoin ( DIH for short ) is a heterocyclic organic compound that is used in organic synthesis as an iodination reagent and as an oxidizing agent.
Extraction and presentation
1,3-Diiodo-5,5-dimethylhydantoin was first produced in 1965 by reacting iodine monochloride with 5,5-dimethylhydantoin in the presence of sodium hydroxide solution . The starting material 5,5-dimethylhydantoin can be synthesized commercially very easily from potassium cyanate , ammonium carbonate and acetone . A more recent synthesis uses the combination of iodine and potassium iodide as a source of iodine and sodium hypochlorite as an oxidizing agent.
properties
1,3-Diiodo-5,5-dimethylhydantoin forms a pale yellow to light brown powder that melts at 192–196 ° C with decomposition. It is storage-stable at −20 ° C. The compound dissolves well in acetone, a reaction taking place at temperatures above 50 ° C. with the formation of iodoacetone . In addition, the fabric is sensitive to light and moisture.
use
1,3-Diiodo-5,5-dimethylhydrandoin is used in organic synthesis as an iodination reagent and oxidizing agent. In an electrophilic iodination, electron-rich aromatics and heteroaromatics can be converted. Both iodine atoms are used here. Compared to the use of elemental iodine , no hydrogen iodide is produced . Aniline is converted to 4-iodoaniline, phenol to 2,4,6-triiodophenol . Primary alcohols can be oxidized to the corresponding nitriles in the presence of ammonia . The corresponding benzonitriles result from substituted benzyl alcohols . In a similar way, primary, secondary and ternary amines , as well as halides and aldehydes, can be converted to nitriles.
Substituted benzaldehydes can be reacted with secondary amines in the presence of 1,3-diiodo-5,5-dimethylhydrandoin in an oxidative amination to give the corresponding benzamides.
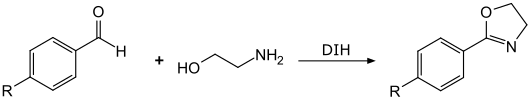
In a similar way, the reaction of benzaldehydes with ethanolamine in the presence of 1,3-diiodo-5,5-dimethylhydrandoin gives rise to oxazolines substituted in the 2-position .
Further substituted heterocycles can be synthesized analogously . The reaction with 1,3-propanediamine gives 1,4,6,6-tetrahydropyrimidines substituted in the 2-position, which can be further oxidized to the corresponding pyrimidines . 3-Phenyl-1-propanol can be cyclized to the chroman .
Individual evidence
- ↑ a b c d e f g h i 1,3-diiodo-5,5-dimethylhydantoin. In: e-EROS Encyclopedia of Reagents for Organic Synthesis . John Wiley and Sons, 1999-2013, accessed December 19, 2017.
- ↑ a b c d e C. Ricco: 1,3-Diiodo-5,5-dimethylhydantoin. In: Synlett . 24, 2013, pp. 2173-2174, doi: 10.1055 / s-0033-1339477 .
- ↑ a b data sheet 1,3-diiodo-5,5-dimethylhydantoin from Sigma-Aldrich , accessed on December 19, 2017 ( PDF ).
- ↑ OO Orazi, RA Corral, HE Bertorello: N-Iodohydantoins. II. Iodinations with 1,3-diiodo-5,5-dimethylhydantoin. In: J. Org. Chem. 30, 1965, pp. 1101-1104, doi: 10.1021 / jo01015a036 .
- ↑ K Mima. Japanese Patent 2013/23475, 2013.
- ↑ VK Chaikovskii, VD Filimonov, AA Funk, VI Skorokhodov, VD Ogorodnikov: 1,3-Diiodo-5,5-dimethylhydantoin — An efficient reagent for iodination of aromatic compound. In: Russian J. Org. Chem. 43, 2007, pp. 1291-1296, doi: 10.1134 / S1070428007090060
- ↑ S. Iida, H. Togo: Oxidative Conversion of Primary Alcohols, and Primary, Secondary, and Tertiary Amines into the Corresponding Nitriles with 1,3-Diiodo-5,5-dimethylhydantoin in Aqueous NH 3 . In: Synlett. 2007, pp. 407-410, doi: 10.1055 / s-2007-967954 .
- ↑ S. Iida, H. Togo: Direct oxidative conversion of alcohols and amines to nitriles with molecular iodine and DIH in aq NH 3 . In: Tetrahedron . 63, 2007, pp. 8274-8281, doi: 10.1016 / j.tet.2007.05.106 .
- ↑ S. Iida, R. Ohmura, H. Togo: Direct oxidative conversion of alkyl halides into nitriles with molecular iodine and 1,3-diiodo-5,5-dimethylhydantoin in aq ammonia. In: Tetrahedron. 65, 2009, pp. 6257-6262, doi: 10.1016 / j.tet.2009.05.001 .
- ↑ H. Baba, K. Moriyama, H. Togo: Preparation of N, N-Dimethyl Aromatic Amides from Aromatic Aldehydes with Dimethylamine and Iodine Reagents. In: Synlett . 23, 2012, pp. 1175-1180, doi: 10.1055 / s-0031-1290659 .
- ↑ S. Takahashi, H. Togo: An Efficient Oxidative Conversion of Aldehydes into 2-Substituted 2-Oxazolines Using 1,3-Diiodo-5,5-dimethylhydantoin. In: Synthesis . 2009, pp. 2329-2332, doi: 10.1055 / s-0029-1216843 .
- ↑ S. Takahashi, H. Togo: Direct Oxidative Conversion of Aldehydes into 2-Substituted 1,4,5,6-Tetrahydropyrimidines Using Molecular Iodine or 1,3-Diiodo-5,5-dimethylhydantoin. In: Heterocycles . 82, 2010, pp. 593-601, doi: 10.3987 / COM-10-S (E) 29 .
- ↑ S. Furuyama, H. Togo: An Efficient Preparation of Chroman Derivatives from 3-Aryl-1-propanols and Related Compounds with 1,3-Diiodo-5,5-dimethylhydantoin under Irradiation Conditions. In: Synlett . 2010, pp. 2325-2329, doi: 10.1055 / s-0030-1258017 .