1-vinylimidazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1-vinylimidazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 6 N 2 | |||||||||||||||
| Brief description |
colorless to pale yellow or brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 94.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.039 g cm −3 (25 ° C ) |
|||||||||||||||
| Melting point |
<−50 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
0.5 hPa (20 ° C) |
|||||||||||||||
| solubility |
miscible with water and alcohols |
|||||||||||||||
| Refractive index |
1.5300 (25 ° C, 589 nm) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
of particular concern : toxic for reproduction ( CMR ) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-Vinylimidazole is a water-soluble basic monomer that forms quaternizable homopolymers and functional copolymers with a variety of vinyl and acrylic monomers through free radical polymerization . N -Vinylimidazole acts as a reactive thinner in UV varnishes, inks and adhesives, as well as a monomer for water-soluble polymers that are used as oilfield chemicals and cosmetic auxiliaries.
Occurrence and representation
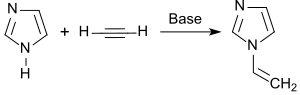
The synthesis and properties of 1-vinylimidazole were described by Walter Reppe in a comprehensive article in 1957. Here, imidazole first with potassium hydroxide solution reacted imidazolate to potassium and the water removed by distillation. Zinc oxide and potassium hydroxide are added to the basic catalyst potassium imidazolate and the free imidazole is ethynylated in 1,4-dioxane at 130 ° C. with acetylene in an autoclave. The yield is 62%.
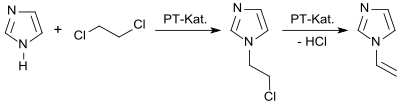
In a laboratory process, imidazole reacts in a two-phase system in the presence of a phase transfer catalyst with 1,2-dichloroethane to form 1- (2-chloroethyl) imidazole and this with elimination of hydrogen chloride in 92% yield to form 1-vinylimidazole.
A specification that can also be used for laboratory quantities specifies the vinylation of imidazole with bromoethene and kieselguhr -geträgertem cesium fluoride in acetonitrile with a yield of 65%.
properties
1-vinylimidazole is a colorless to brown, light-sensitive, hygroscopic and slightly alkaline liquid with an unpleasant amine odor reminiscent of rotting fish. The compound is very soluble in water and alcohols. The radical polymerization of 1-vinylimidazole proceeds only very slowly at pH 9, but as fast at pH 1 as that of quaternized 1-vinylimidazole.
1-vinylimidazole has a flash point of 84 ° C, a decomposition temperature of 220 ° C and an ignition temperature of 415 ° C.
Applications
Because of its high reactivity for radical (UV) polymerization, 1-vinylimidazole is used as a so-called reactive thinner in UV varnishes, inks and adhesives for coatings and varnishes as well as for the functionalization of polymer surfaces by UV-induced grafting to improve wettability and the Adhesion used.
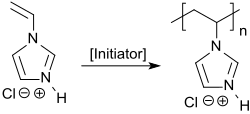
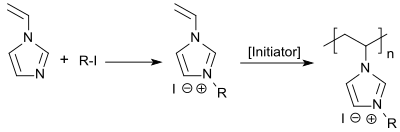
1-vinylimidazole can be quaternized with n -alkyl iodides to give 3- n -alkyl-1-vinylimidazolium iodides and with dimethyl sulfate to give 3-methyl-1-vinylimidazolium methosulfate. The quaternary ammonium compounds obtained can be polymerized radically in aqueous solution with the water-soluble azo initiator 4,4'-azobisvaleric acid.
Copolymers of quaternary N- vinylimidazolium salts and polar monomers, in particular N- vinylpyrrolidone, are cationic polyelectrolytes and are, inter alia, suitable. a. as a flocculant for water treatment, as a flotation aid for coal and ore processing, as an additive for drilling fluids and cementing in oil production, as an emulsion breaker for the dewatering of crude oil emulsions in refineries, and as corrosion inhibitors for iron alloys.
Copolymers of quaternary N- vinylimidazolium salts and polymerizable unsaturated carboxylic acids, such as. B. methacrylic acid or sulfonic acids , such as. B. 2-acrylamido-2-methylpropanesulfonic acid reduce the electrostatic charge, e.g. B. of hair and are therefore used in shampoos to improve wet combability.
1-Vinylimidazole polymerizes radically in aqueous or alcoholic solution to give homopolymers with average molar masses of 2,000 to 50,000, which, however, often still contain relatively high residual monomer contents (> 600 ppm). By adding sulfur-containing regulators, such as. B. mercaptoethanol , the undesirable residual content of the malodorous N- vinylimidazole can be reduced to less than 50 ppm, although the molecular weight of the polymer obtained also decreases.
Hydrogels made from poly-1-vinylimidazole very efficiently bind a large number of heavy metal ions (except for Pb 2+ ), which can be eluted again selectively and quantitatively from the hydrogel.
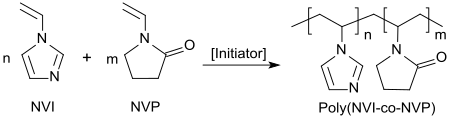
The 1-vinylimidazole itself can be free radical copolymerized with a variety of vinyl and acrylic monomers. Water-soluble copolymers with vinyl pyrrolidone are used as color transfer inhibitors in detergent preparations,
with vinyl acetate as a coating for lithographic printing plates , with acrylic acid esters or methacrylic acid esters or 2-hydroxyethyl methacrylate as an adhesion promoter in paints or with acrylonitrile as a precursor for carbon fibers .
Individual evidence
- ↑ a b Entry on 1-vinylimidazole at TCI Europe, accessed on April 5, 2017.
- ↑ a b c d data sheet 1-vinylimidazole from Sigma-Aldrich , accessed on December 29, 2019 ( PDF ).
- ↑ a b c d e f Entry on 1-vinylimidazole in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c Patent EP0698046B1 : Homo- and copolymers of 1-vinylimidazole, process for their preparation and their use. Applied on May 2, 1994 , published on March 5, 1997 , applicant: BASF AG, inventor: J. Detering, W. Denzinger.
- ^ Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Amsterdam, NL 2014, ISBN 978-0-323-28659-6 , pp. 67 .
- ↑ Entry on 1-vinylimidazole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 29, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on June 26, 2020.
- ^ W. Reppe: vinylation . In: Justus Liebigs Ann. Chem. Band 601 , no. 1 , 1957, pp. 81-138 , doi : 10.1002 / jlac.19566010106 .
- ↑ D. Bogdal, K. Jaskat: Synthesis of vinyl monomers with active azaaromatic groups. Phase transfer catalytic approach . In: Synth. Commun. tape 30 , no. 18 , 2000, pp. 3341-3352 , doi : 10.1080 / 00397910008086974 .
- ↑ S. Hayat et al .: N-Alkylation of anilines, carboxamides and several nitrogen heterocycles using CsF-Celite / alkyl halides / CH3CN combination . In: Tetrahedron . tape 57 , no. 50 , 2001, p. 9951-9957 , doi : 10.1016 / S0040-4020 (01) 00989-9 .
- ↑ S. Santanakrishnan, RA Hutchinson: Free-radical polymerization of N-vinylimidazole and quaternized vinylimidazole in aqueous solution . In: Macromol. Chem. Phys. tape 214 , no. 10 , 2013, p. 1140–1146 , doi : 10.1002 / macp.201300044 .
- ↑ JC Salamone, SC Israel, P. Taylor, B. Snider: Synthesis and homopolymerization studies of vinylimidazolium salts . In: polymer . tape 14 , no. 12 , 1973, p. 639-644 , doi : 10.1016 / 0032-3861 (73) 90039-6 .
- ↑ Patent EP0544158A1 : Use of homo- and copolymers based on quaternized 1-vinylimidazoles as organic polyelectrolytes. Applied on November 26, 1991 , published on June 2, 1993 , applicant: BASF AG, inventor: H. Meyer, A. Sanner, R.-D. Reinhardt, F. Frosch, H.-J. Raubenheimer.
- ↑ Patent US6355231B1 : Use of cationic copolymers of unsaturated acids and N-vinylimidazolium salts in cosmetic hair formulations. Registered on October 8, 1998 , published on March 12, 2002 , applicant: BASF AG, inventor: R. Dieing, P. Hössel, A. Sanner.
- ↑ Patent DE2814287 : detergent with a content of discoloration-inhibiting additives. Registered on April 3, 1978 , published on October 11, 1979 , applicant: Henkel KGaA, inventors: H. Waldhoff, E. Schmadel, K. Engelskirchen, J. Galinke.
- ↑ BL Rivas, HA Maturana, MJ Molina, MR Gómez-Aaantón, IF Piérola: Metal ion binding properties of poly (N-vinylimidazole) hydrogels . In: J. Appl. Polym. Sci. tape 67 , no. 6 , 1998, pp. 1109-1118 , doi : 10.1002 / (SICI) 1097-4628 (19980207) 67: 6 <1109 :: AID-APP19> 3.0.CO; 2-V .
- ↑ Patent US6172027B1 : Use of watersoluble copolymers comprising N-vinylimidazole units as color transfer inhibitors in detergents. Applied on December 4, 1997 , published on January 9, 2001 , applicant: BASF AG, inventor: D. Boeckh, S. Stein, A. Funhoff, JA Lux, H.-U. Hunter.
- ↑ Patent US6649323B2 : Overcoat for light-sensitive materials comprising (1-vinylimidazole) polymer or copolymer. Applied January 21, 2000 , published November 18, 2003 , Applicant: Kodak Polychrome Graphics LLC, Inventors: SP Peppas, H. Baumann, U. Dwars, CM Savariar-Hauck, H.-J. Timpe.
- ↑ Patent US8512465B2 : Use of copolymers as adhesion promotors in lacqueurs. Registered on November 15, 2007 , published on August 20, 2013 , applicant: BYK-Chemie GmbH, inventors: K. Haubennestel, S. Mossmer, T. Launag, A. Frank.
- ^ W. Deng: Poly (acrylonitrile-co-1-vinylimidazole): A new melt processable carbon fiber precursor . Ed .: Clemson University, Tiger Prints. Clemson 2010 ( online ).