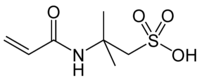
2-acrylamido-2-methylpropanesulfonic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-acrylamido-2-methylpropanesulfonic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 13 NO 4 S | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 207.25 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.1 g cm −3 (15.6 ° C) |
|||||||||||||||
| Melting point |
185 ° C |
|||||||||||||||
| solubility |
very easily soluble in water (1500 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2-Acrylamido-2-methylpropanesulfonic acid (trademark of Lubrizol : AMPS ) is a chemical compound from the group of sulfonic acids . The compound, also known as 2-acrylamido- tert- butylsulfonic acid (ATBS), is a very reactive and readily water-soluble acrylic monomer that forms high-molecular homopolymers ( molecular weight > 2 million) with exceptional product properties and by means of radical copolymerization with other monomers for the targeted hydrophilicization of the obtained Copolymers is used. Originally proposed to improve the dyeability of polyacrylonitrile fibers , AMPS has found wide use in a large number of applications due to its functionality.
Occurrence
It is only made synthetically. A natural occurrence is not known.
Manufacturing
2-Acrylamido-2-methylpropanesulfonic acid is produced in a Ritter reaction from acrylonitrile and isobutene in the presence of highly concentrated or fuming sulfuric acid and water. The more recent patent literature describes discontinuous and continuous processes that produce AMPS in high purity (up to 99.7%) and improved yield (up to 89%, based on isobutene) when liquid isobutene is added to an acrylonitrile / sulfuric acid / phosphoric acid mixture at 40 ° C .
properties
AMPS is a white, crystalline, hygroscopic solid that dissolves very well in water and forms easily soluble salts with alkali and alkaline earth metals. Aqueous AMPS solutions react strongly acidic because of the complete dissociation of the sulfonic acid group. The solid in powder and granulate form and aqueous 50% solutions of the sodium (AMPS-Na) and ammonium salts are commercially available. As an acrylic monomer, AMPS has unusual material properties that make it particularly interesting as a comonomer in functional polymers:
- High thermal and hydrolytic stability: The amide function in the AMPS molecule is spatially shielded by the geminal methyl groups and the sulfomethyl group and protected against hydrolytic and thermal degradation. The decomposition of AMPS homopolymers takes place from 225 ° C, of AMPS-Na homopolymers even only from 307 ° C.
- Pronounced hydrophilicity and acidity : The sulfonic acid function gives AMPS a permanently hydrophilic character and is present as a sulfonate anion well into the acidic pH range. Even small amounts of AMPS (<5% by weight) increase the hydrophilicity of copolymers considerably.
- Good solubility: AMPS dissolves very well in water and well in the aprotic-dipolar solvents dimethylformamide and N- methyl-2-pyrrolidone .
The high stability of the AMPS results in its low biodegradability. It is not mutagenic and, as a neutral AMPS Na salt solution, has a very low toxicity LD 50 > 16,000 mg / kg rat.
It polymerizes easily, even under the influence of light .
use
As a component of copolymers with acrylic acid , acrylamide and other functional acrylic and vinyl monomers. AMPS has a number of uses as an additive or comonomer in:
1. Textile: increases the dyeability of polyacrylic and polyester-containing textiles.
2. Paints, varnishes and paper coating: causes a higher chemical and mechanical stability of polymer emulsions, stabilizes the particle distribution, suppresses the formation of grits and increases the abrasion resistance of paints and papers.
3. Adhesives: improves the thermal and mechanical properties and increases the bond strength of pressure-sensitive adhesive formulations.
4. Detergent: increases the washing effect of surfactants by binding multivalent cations and reduces the accumulation of dirt.
5. Cosmetics and medicine: forms high molecular weight polymers with other comonomers, which are used as hydrogels and in emulsions as thickeners, stabilizers, dispersants and lubricants in cosmetic, dermatological and medical preparations. Because of its high water absorption and retention capacity, it is used as a comonomer in superabsorbents for e.g. B. used baby diapers.
6. Water treatment: stabilizes polyvalent cations in hard water, especially in copolymers and terpolymers with other ionic and neutral monomers, and acts as a 'scale inhibitor' to prevent scale formation and corrosion.
7. Plant protection: increases the bioavailability of plant protection agents in aqueous-organic preparations in dissolved and nanoparticulate polymer preparations.
8. Membranes: increases water flow, retention and fouling resistance of asymmetric ultrafiltration and microfiltration membranes and is being investigated as an anionic component in polymeric fuel cell membranes.
9.Building: acts in copolymers as a water retention agent in mortars and plasters, leads to increasing dispersibility of particles, increases the slip and improves the mechanical and chemical stability of highly flowable and self-compacting concrete in copolymers, especially with methacrylic or acrylic acid , as a concrete plasticizer .
10. Oil and gas exploration: increases the stability of polymers in oil and gas field applications to high temperatures and pressures, as well as high salt concentrations, reduces friction and fluid loss in drilling and cementing fluids and is used in tertiary oil extraction and hydraulic fracturing called breaking up of rock formations for the production of shale gas .
The current production capacity of the three main AMPS manufacturers is over 30,000 tons / year.
Individual evidence
- ↑ Entry on 2-ACRYLAMIDO-2-METHYLPROPANE SULFONIC ACID in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ a b c d Data sheet 2-Acrylamido-2-methylpropanesulfonic acid (PDF) from Merck , accessed on January 19, 2011.
- ↑ a b Entry on 2-acrylamido-2-methylpropanesulfonic acid in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on 2-acrylamido-2-methylpropanesulfonic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Vinati Organics Ltd: 2-Acrylamido 2-methylpropanesulfonic Acid (ATBS) ( Memento from October 30, 2010 in the Internet Archive )
- ↑ Patent US3506707 : Preparation of acrylamidoalkanesulfonic acids. Filed February 1, 1968 , published April 14, 1970 , applicant: Lubrizol Corp., inventor: Leonard E Miller, Donald L Murfin.
- ↑ US 6,504,050, inventor: PP Barve et al., Applicant: CSIR (IN), published January 7, 2003
- ↑ WO Parker, Jr., A. Lezzi, Polymer, 34 (23), 4913-4918 (1993)
- ↑ a b Lubrizol, AMPS (R) Specialty Monomers, Oil Field Applications
- ^ The Lubrizol Corp .: HPV Challenge Program, Test Plan for AMPS (PDF; 1.1 MB), Aug. 1, 2000.
- ↑ Vinati Organics Ltd., Product Data Sheet
- ↑ EP 1 611 278, inventor: W. Brennich et al., Applicant: CHT R. Beitlich GmbH, issued on January 24, 2007
- ^ GP Marks, AC Clark, ACS Symposium Series, 775 , (5), 46-53 (2000) and EP 0 973 807, inventor: R. Figge, H.-P. Weitzel, applicant: Wacker-Chemie GmbH, published on September 20, 2000
- ↑ US 4,012,560, inventor: JC Baatz, AE Corey, applicant: Monsanto Co., issued March 15, 1977 and WO 2007/057333, inventor: A. Hashemzadeh, applicant: Wacker Polymer Systems, published May 24, 2007
- ↑ US 7,928,047, inventor: M.-S. Cho, Applicant: LG Household & Health Care Ltd., published April 19, 2011
- ↑ EP 1 236 464, inventor: M. Löffler et al., Applicant: Clariant GmbH, published on September 4, 2002 and EP 2 055 315, inventor: R. von Eben-Worlée et al., Applicant: Worlée-Chemie, published on May 6, 2009
- ↑ WO 2011/131526, inventor: N. Herfert et al., Applicant: BASF SE, published on October 27, 2011
- ^ Z. Amjad, Tenside Surf. Det., 44 (4), 202-208 (2007)
- ↑ Patent US20110166309 : Preparation containing at least one type of fungicidal conazole. Filed on March 14, 2011 , published on July 7, 2011 , Applicant: BASF , inventor Sebastian Koltzenburg et al ..
- ↑ EP 1 681 923, inventor: S. Koltzenburg et al., Applicant: BASF AG, published on April 20, 2011
- ↑ US 6,183,640, inventor: I. Wang, applicant: USF Filtration and Separations Group, Inc., issued February 6, 2001
- ↑ US 2008/020255, inventors: H. Hiraoka, T. Yamaguchi, applicants: Toagosei Co., Ltd., published January 24, 2008 and H. Diao et al., Macromolecules, 43 (15), 6398-6405 ( 2010)
- ↑ EP 0 936 228, inventor: G. Albrecht et al., Applicant: SKW Trostberg AG, published on August 18, 1999 and WO 03/085013, inventor: C. Spindler et al., SKW Polymers GmbH, published on August 16, 1999 October 2003
- ^ "Modern Superplasticizers in Concrete Technology", Verein Deutsche Bauchemie eV, Frankfurt am Main, January 2007 and Y.-S. Ye et al., J. Appl. Polym. Sci., 100 , (3), 2490-2496 (2006) and CT Liao et al., Cement and Concrete Research, 36 , (4), 650-655 (2006) and A. Buyukyagaci et al., Cement and Concrete Research, 39 , (7), 629-635 (2009)
- ↑ US 6,448,311, inventor: ML Walker, applicant: Baker Hughes Inc., issued September 10, 2002
- ↑ Patent US4573533 : Enhanced oil recovery. Filed June 21, 1984 , published March 4, 1986 , Applicant: American Cyanamid Company, Inventor: Roderick G. Ryles, Albert G. Robustelli. And A. Sabhapondit et al., J. Appl. Polym. Sci., 87 , (12), 1869-1878 (2003)
- ↑ Patent US20070204996 : Fracturing a subterranean formation using an aqueous treatment fluid including a friction-reducing cationic polymer and an oxidizer as an anticoagulant (crosslinking, gelation inhibition) to prevent flocculation in the well bore. Filed on March 3, 2006 , published on 6 September 2007 , Applicant: Halliburton Energy Services , inventor David McMechan et al .. and US 20100048430, inventor GP Funkhouser, D. Loveless, Applicant: Halliburton Energy Services, Inc. , published February 25, 2010
- ↑ valuepickr.com: Vinati-organics .

