2,3-butanediol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
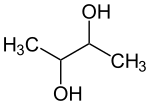
|
||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,3-butanediol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 10 O 2 | |||||||||||||||
| Brief description |
slightly viscous, colorless, almost odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 90.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.01 g cm −3 |
|||||||||||||||
| Melting point |
19 ° C |
|||||||||||||||
| boiling point |
180 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
miscible with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,3-Butanediol is an aliphatic alcohol with two hydroxyl groups (-OH) and the empirical formula C 4 H 10 O 2 .
The arrangement of the hydroxyl groups can be spatially different. There are therefore three stereoisomers .
Occurrence
2,3-butanediol is formed e.g. B. during the brewing process by metabolizing the diacetyl by the brewing yeast. Top-fermented beers can contain 2,3-butanediol in a concentration between 50 and 130 mg / l.
Extraction and presentation
2,3-Butanediol is obtained from corn sugar through acid hydrolysis . With biotechnological methods (conversion with bacteria which are capable of 2,3-butanediol fermentation , e.g. Klebsiella aerogenes (formerly Enterobacter aerogenes )) the individual enantiomers can also be represented in pure form.
properties
Stereoisomerism
2,3-Butanediol is chiral and contains two equally substituted stereogenic centers, so there are three stereoisomers : ( R , R ) -2,3-butanediol [synonym: D - (-) - 2,3-butanediol] and the enantiomeric one ( S , S ) -2,3-butanediol [synonym: L - (+) - 2,3-butanediol] and meso -2,3-butanediol.
The physical properties such as the melting or boiling point of the three isomeric 2,3-butanediols differ and, in the case of mixtures of the isomers, vary with their composition.
| 2,3-butanediols | ||||
| Surname | D - (-) - 2,3-butanediol | L - (+) - 2,3-butanediol | meso -2,3-butanediol |
DL -2,3-butanediol ( racemate from D- and L -form) |
| Structural formula |
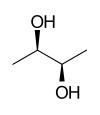
|

|

|

|
| other names | (2 R , 3 R ) -2,3-butanediol | (2 S , 3 S ) -2,3-butanediol | rac -2,3-butanediol | |
| CAS number | 24347-58-8 | 19132-06-0 | 5341-95-7 | 513-85-9 |
| Melting point | 25 ° C | 19.7 ° C | 32-34 ° C | 7.6 ° C |
| boiling point | 180-182 ° C | 178-181 ° C | 183-184 ° C | 182 ° C |
| Density in g / cm³ | 0.9872 (25 ° C) | 0.9869 (25 ° C) | 1,0003 (20 ° C) | 1.0033 (20 ° C) |
| Refractive index at 25 ° C | 1.4306 | 1.4340 | 1.4367 | 1.4310 |
| Rotation value [α] D 25 ° C | + 12.5 ° (undiluted) | −13 ° (undiluted) | ± 0 ° | ± 0 ° |
Safety-related parameters
2,3-Butanediol forms flammable vapor-air mixtures at high temperatures. The compound has a flash point of 85 ° C. The explosion range is between 3.1 vol.% As the lower explosion limit (LEL) and 11.4 vol.% As the upper explosion limit (UEL). The ignition temperature is 400 ° C. The substance therefore falls into temperature class T2.
use
2,3-Butanediol is mostly used as a substitute for glycerine . It is also used as a solvent , mostly for paints and as a raw material for the production of butadiene , plastics and epoxy resins . In toiletries, it is used as an additive in hair tonics, shampoos and fixatives, as well as in shaving foam and toothpaste.
Individual evidence
- ↑ Entry on 2,3-BUTANEDIOL in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ a b c d e f g h i j k l Entry on 2,3-butanediol in the GESTIS substance database of the IFA , accessed on May 29, 2018(JavaScript required) .
- ↑ Data sheet 2,3-butanediol (PDF) from Merck , accessed on January 18, 2011.
- ^ CRC Handbook of Chemistry and Physics , 60th Edition, CRC Press, 1980.
- ^ A b c E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Chemielexikon, Hermann Römpp, Fifth Edition, Franckh'sche Verlagshandlung, 1962.