2,4-pentanediol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,4-pentanediol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 O 2 | |||||||||||||||
| Brief description |
colorless to yellowish odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.96 g cm −3 |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.435 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2,4-Pentanediol is a chemical compound from the group of alkanediols that occurs in three isomeric forms. It can still be in the meso form and as a racemate .
Isomerism
2,4-Pentanediol is a chiral compound with two stereocenters . Consequently, there are the two enantiomeric forms ( R, R ) -2,4-pentanediol and ( S, S ) -2,4-pentanediol and the achiral meso compound ( R, S ) -2,4-pentanediol.
| Isomers of 2,4-pentanediol | |||
| Surname | ( R, R ) -2,4-pentanediol | ( S, S ) -2,4-pentanediol | meso-2,4-pentanediol |
| other names | (-) - 2,4-pentanediol | (+) - 2,4-pentanediol | ( R *, S * ) -2,4-pentanediol |
| Structural formula |  |
 |
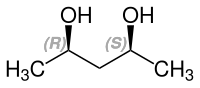
|
| CAS number | 42075-32-1 | 72345-23-4 | 3950-21-8 |
| 625-69-4 (unspec.) | |||
| PubChem | 2723683 | 6950200 | 6950199 |
| 12262 (unspec.) | |||
| Wikidata | Q72499966 | Q97731988 | Q27287604 |
| Q27271503 (unspec.) | |||
Extraction and presentation
2,4-Pentanediol can be obtained by asymmetric hydrogenation of 2,4-pentanedione .
properties
2,4-Pentanediol is a flammable, hardly inflammable, colorless to yellowish, odorless liquid that is miscible with water.
use
2,4-Pentanediol can be used to synthesize chelated polynuclear complexes. The R form is used as an acetalizing reagent for ketones and β-ketoesters, in the synthesis of optically active polyesters and as a diol with a wide range of possible uses as a chiral auxiliary, building block and chiral ligand.
Individual evidence
- ↑ a b c d e f g h i j Entry on 2,4-pentanediol in the GESTIS substance database of the IFA , accessed on December 2, 2018(JavaScript required) .
- ↑ a b Data sheet (R, R) - (-) - 2,4-Pentanediol, 99% from Sigma-Aldrich , accessed on December 2, 2018 ( PDF ).
- ↑ Data sheet (2S, 4S) - (+) - Pentanediol, 99% from Sigma-Aldrich , accessed on December 2, 2018 ( PDF ).
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 454 ( limited preview in Google Book search).
- ↑ a b c data sheet 2,4-pentanediol, 98% from Sigma-Aldrich , accessed on December 2, 2018 ( PDF ).
- ^ JG Pritchard, RL Vollmer: The meso and Racemic Forms of 2,4-Pentanediol and Certain of Their Derivatives. In: The Journal of Organic Chemistry. 28, 1963, p. 1545, doi : 10.1021 / jo01041a025 .
- ↑ J. Michael Chong: (2R, 4R) -2,4-pentanediol . In: Encyclopedia of Reagents for Organic Synthesis, 2001. doi : 10.1002 / 047084289X.rp029