2-aminobenzimidazole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2-aminobenzimidazole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 7 N 3 | ||||||||||||||||||
| Brief description |
white to brown powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 133.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.1873 g cm −3 at 25 ° C |
||||||||||||||||||
| Melting point | |||||||||||||||||||
| boiling point |
369 ° C |
||||||||||||||||||
| solubility |
Slightly soluble in water (<1 g cm −3 at 20 ° C ), soluble in ethanol and acetone , little soluble in diethyl ether , benzene and DMSO |
||||||||||||||||||
| Refractive index |
1.5341 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2-aminobenzimidazole is a chemical compound that consists of a benzene ring and a fused imidazole ring and bears an amino group in the 2-position (i.e. between the two nitrogen atoms of the imidazole) . This functional bicyclic heteroaromatic is the starting material of a variety of physiologically active substances, from fungicides and worms to also against problem germs such. B. Pseudomonas aeruginosa , active ingredients.
Occurrence and representation
The production of 2-aminobenzimidazole was first reported in 1908 from the extremely toxic cyanogen bromide and 1,2-phenylenediamine .
The synthesis with cyanamide (instead of cyanogen bromide) in aqueous solution is much better suited , from which 2-aminobenzimidazole precipitates in 92% yield in light brown flakes.
Further synthesis variants are described, but are more suitable for derivatives substituted on the benzene ring on a laboratory scale.
properties
2-aminobenzimidazole is a poorly water-soluble solid which crystallizes in pure form in white flakes. In polar solvents, such as. B. in acetone and ethanol, or in alkali it dissolves easily.
The tautomerism Equilibrium is not on the side of the French against the expressed in the first publication in 1908 conjecture. orthophénylèneguanidine called imide , but far on the part of the amine 2-aminobenzimidazole.
1 H -benzimidazol-2-amine also appears in the soil as a bacterial breakdown product of the fungicides carbendazim and benomyl (which are obsolete in the EU) .
Applications
As already reported in the first publication in 1908, HNO 2 is formed from 1 H -benzimidazol-2-amine by diazotization with nitrous acid and subsequent boiling, benzimidazolone (hydroxybenzimidazole, here referred to as "orthophénylèneurée").
The reaction of 2-aminobenzimidazole with hydroxylamine-O-sulfonic acid HOSA gives 1,2-diaminobenzimidazole (75% yield),
which has anti- corrosive properties and whose derivatives have been investigated for their suitability as antibacterial and antifungal drugs.
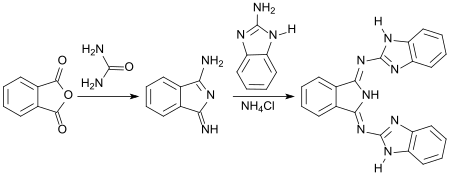
With the 1,3-diiminoisoindoline and 2-aminobenzimidazole accessible from phthalic anhydride and urea , a yellow dye is obtained, e.g. B. for polyester fibers.
With dicarbonyl compounds, 2-aminobenzimidazole forms easily condensed heterocycles . So z. B. with acetylenedicarboxylic acid dimethyl ester a dihydropyrimido [1,2-a] benzimidazole.
2-aminobenzimidazole is a structural element of a large number of functional chemicals in the field of fungicides , such as. B. Carbendazim, Benomyl, from anthelmintics such. B. mebendazole , fenbendazole , oxfendazole , albendazole and flubendazole , as well as antibiotics against multi-resistant MRSA germs.
A common route for building these compounds is the synthesis of a (usually in the 5-position) modified 1,2-phenylenediamine, whose ring closure with cyanamide to the corresponding 2-aminobenzimidazole and subsequent derivatization at the amino group to the end product, such. B. with the anthelmintic albendazole.
Individual evidence
- ↑ a b c Entry on 2-aminobenzimidazole at TCI Europe, accessed on May 30, 2020.
- ↑ a b c d e f Data sheet 2-aminobenzimidazole for synthesis (PDF) from Merck , accessed on May 30, 2020.
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 149 .
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4822-6097-7 , pp. 3-38 .
- ↑ a b R. Rastogi, S. Sharma: 2-Aminobenzimidazole in Organic Syntheses . In: Synthesis . tape 11 , 1983, pp. 861-882 , doi : 10.1055 / s-1983-30546 .
- ^ R. Frei, AS Breitbach, HE Blackwell: 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms . In: Angew. Chem. Int. Ed. tape 51 , no. 21 , 2012, p. 5226-5229 , doi : 10.1002 / anie.201109258 .
- ↑ P. Pierron: Sur les cyanamides aromatiques monoatomiques . In: Ann. Chim. Phys. tape 15 , no. 8 , 1908, pp. 189-193 ( bnf.fr ).
- ↑ S. Weiss, H. Michaud, H. Prietzel, H. Krommer: New, simple synthesis of 2-aminobenzimidazole . In: Angew. Chem. Band 85 , no. 19 , 1973, p. 866-867 , doi : 10.1002 / anie.19730851910 .
- ^ J. Backes, B. Heinz, WG Ried: Houben-Weyl: Methods of Organic Chemistry, 4th edition . E 8c. Thieme, Stuttgart 1994, ISBN 978-3-13-797804-6 , p. 219 .
- ^ Gerhard Eisenbrand, Alfred Hagen Meyer, Peter Schreier: RÖMPP Lexikon Lebensmittelchemie, 2nd edition, Thieme Verlag, Stuttgart 2006, ISBN 978-3-13-736602-7 , p. 405 .
- ↑ P. Pierron: Sur les cyanamides aromatiques monoatomiques . In: Ann. Chim. Phys. tape 15 , no. 8 , 1908, pp. 195 ( bnf.fr ).
- ↑ D. Vlaović, J. Čanadanović-Brunet, J. Balaž, I. Juranić, D. Djoković, K. Mackenzie: Synthesis, AntiBacteriol, and Antifungal Activities of Some New Benzimidazoles . In: Biosci. Biotech. Bioch. tape 56 , no. 2 , 1992, p. 199–206 , doi : 10.1271 / bbb.56.199 .
- ↑ Patent US3499908 : Production of 1,3-bis (heterocycloimino) -isoindolines from 3-iminoisoindolenines and heterocyclic amines. Registered on November 14, 1966 , published on March 10, 1970 , applicant: Farbenfabriken Bayer AG, inventor: H. Vollmann, H. Leister.
- ↑ RW Huisgens III, S. Reyes, CS Reed, C. Bunders, SA Rogers, AT Steinhauer, C. Melander: The chemical synthesis and antibiotic activity of a diverse library of 2-aminobenzimidazole small molecules against MRSA and multidrug-resistant A. baumannii . In: Bioorg. Med. Chem. Band 18 , no. 2 , 2010, p. 663-674 , doi : 10.1016 / j.bmc.2009.12.003 .
- ^ Ruben Vardanyan, Victor Hruby: Synthesis of Essential Drugs . Elsevier Science, 2006, ISBN 978-0-444-52166-8 , pp. 585 .