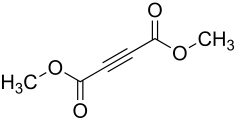
Acetylenedicarboxylic acid dimethyl ester
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Acetylenedicarboxylic acid dimethyl ester | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 6 O 4 | |||||||||||||||
| Brief description |
slightly yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 142.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.16 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−18 ° C |
|||||||||||||||
| boiling point |
195-198 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| Refractive index |
1.447 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Acetylenedicarboxylic acid dimethyl ester (DMAD) is an organic chemical compound with the formula C 6 H 6 O 4 from the group of carboxylic acid esters with a C≡C triple bond.
Extraction and presentation
Maleic acid is brominated and the resulting dibromo succinic acid with potassium hydroxide dehydrohalogenated. The resulting acetylenedicarboxylic acid is then esterified with methanol and sulfuric acid as a catalyst .
properties
Acetylenedicarboxylic acid dimethyl ester is a pale yellowish liquid that decomposes when heated or when exposed to light.
use
As an alkyne that is liquid at room temperature, DMAD is highly electrophilic . It is therefore used as a dienophile in cycloaddition reactions - such as the Diels-Alder reaction . It is also a strong Michael acceptor .
safety instructions
Acetylenedicarboxylic acid dimethyl ester is an irritant and a corrosive agent . Contact with metals can produce hydrogen, which creates a risk of explosion.
Individual evidence
- ↑ a b c d e f g h i data sheet for dimethyl acetylenedicarboxylate (PDF) from Merck , accessed on March 2, 2013.
- ↑ a b Datasheet Dimethyl acetylenedicarboxylate from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ Data sheet Dimethyl Acetylenedicarboxylate (PDF) from Fisher Scientific , accessed on February 13, 2014.

