3,6-dibromopyrocatechol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
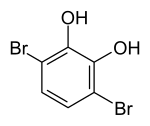
|
|||||||||||||
| General | |||||||||||||
| Surname | 3,6-dibromopyrocatechol | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 6 H 4 Br 2 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 267.9 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
83-84 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
3,6-Dibromopyrocatechol is a chemical compound that belongs to both the phenols and the bromoaromatics. It is isomeric to 3,4-dibromopyrocatechol , 3,5-dibromopyrocatechol and 4,5-dibromopyrocatechol .
presentation
The synthesis of 3,6-dibromopyrocatechol starts from cyclohexanone , which is reacted with a large excess of copper (II) bromide . 3,6,6-Tribromo-2-hydroxycyclohex-2-en-1-one is formed as an intermediate. The second reaction step is the reaction with aromatization of the ring with lithium carbonate in DMF
Reactions
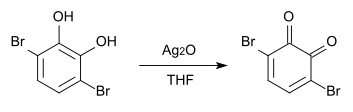
With silver (I) oxide in THF , 3,6-dibromo- o -benzoquinone is formed.
The bromination with potassium bromide and bromine produces tetrabromo-catechol , which has a melting point of 192 ° C.
Individual evidence
- ↑ a b c d J. Paquet, P. Brassard: Reactions of polar dienes with o -quinones . In: Canadian Journal of Chemistry . 67 (8), 1989, pp. 1354-1358, doi : 10.1139 / v89-207 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Kyoko Nishizawa, J. Yasuo Satoh: The Reaction of Cycloalkanones with Copper (II) Halides. II. The Reaction of Cyclohexanones with Copper (II) Bromide. In: Bull. Chem. Soc. Yep 1975, 48, 6, pp. 1875-1877, doi : 10.1246 / bcsj.48.1875 .
- ↑ Kyoko Nishizawa, J. Yasuo Satoh: A Convenient Synthesis of Substituted Pyrocatechols. In: Bull. Chem. Soc. Yep 1975 , 48 , pp. 2215-2216, doi : 10.1246 / bcsj.48.2215 .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.