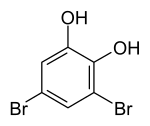
3,5-dibromopyrocatechol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | 3,5-dibromopyrocatechol | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 6 H 4 Br 2 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 267.9 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
58-60 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
3,5-Dibromopyrocatechol is a chemical compound that belongs to both the phenols and the halogen aromatic compounds . It is isomeric to 3,4-dibromopyrocatechol , 3,6-dibromopyrocatechol, and 4,5-dibromopyrocatechol .
presentation
3,5-dibromopyrocatechol can be produced by dakin oxidation from 3,5-dibromosalicylaldehyde , which in turn is synthesized by bromination of salicylaldehyde with elemental bromine in glacial acetic acid .
Reactions
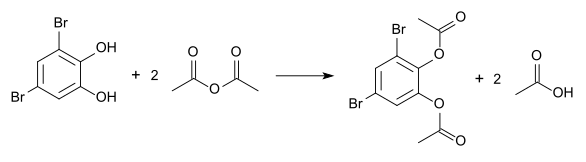
Esterification with acetic anhydride gives the diacetate, the melting point of which is 96 ° C.
3,5-Dibromo- o -benzoquinone is formed with silver oxide in THF .
Analytical evidence
The bromination with potassium bromide and bromine produces tetrabromo-catechol , which has a melting point of 192 ° C.
The 2-methyl ether is registered under the CAS number 79893-93-3, its melting point is 67–68 ° C.
Individual evidence
- ^ A b c Buckingham: Dictionary of organic compounds. Volume 9, p. 1914 ( limited preview in Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b J. Paquet, P. Brassard: Reactions of polar dienes with o -quinones . In: Canadian Journal of Chemistry . 67 (8), 1989, pp. 1354-1358, doi : 10.1139 / v89-207 .
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.