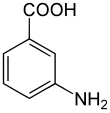
3-aminobenzoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 3-aminobenzoic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 7 H 7 NO 2 | |||||||||||||||||||||
| Brief description |
beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 137.14 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.51 g cm −3 |
|||||||||||||||||||||
| Melting point |
174 ° C |
|||||||||||||||||||||
| pK s value |
3.07; 4.79 |
|||||||||||||||||||||
| solubility |
poor in water (5.9 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
3-aminobenzoic acid is an organic carboxylic acid that is used to make azo dyes . In addition to the 3-aminobenzoic acid, two further position exist isomeric forms: the anthranilic acid ( o -aminobenzoic acid) and the 4-aminobenzoic acid ( p -aminobenzoic acid ).
Extraction and presentation
3-aminobenzoic acid can be obtained by the reduction of 3-nitrobenzoic acid . Elemental zinc in hydrochloric acid or hydrazine , for example, are suitable as reducing agents .
toxicology
3-aminobenzoic acid shows low toxicity. In addition, no indications of reproductive toxicity, mutagenicity or carcinogenicity were found.
Web links
Individual evidence
- ↑ a b c d e f g Entry on 3-aminobenzoic acid in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ CRC Handbook of Chemistry and Physics , 85th Edition, CRC Press, Boca Raton, 2004.
- ↑ Data sheet 3-aminobenzoic acid from Sigma-Aldrich , accessed on November 8, 2008 ( PDF ).
- ↑ J. Wilbrand , FK Beilstein : About a new series of isomeric compounds of the Benzoë group. - Nitrodracylic acid and its derivatives , in: J. Liebigs Ann. Chem. , 1863, 128 , pp. 257-273; doi : 10.1002 / jlac.18631280302 , ( PDF ).
- ^ T. Curtius : The action of hydrazine hydrate on nitro compounds. In: J. für Prakt. Chemie 184, 233-237 (1907), digitized version on Gallica .
