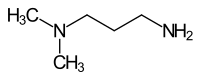
3-aminopropyldimethylamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-aminopropyldimethylamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 14 N 2 | |||||||||||||||
| Brief description |
colorless to yellowish liquid with an amine-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 102.18 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.82 g cm −3 |
|||||||||||||||
| Melting point |
<−70 ° C |
|||||||||||||||
| boiling point |
134 ° C |
|||||||||||||||
| Vapor pressure |
6 mbar (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.435 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-aminopropyldimethylamine is a chemical compound from the group of aliphatic amines .
Extraction and presentation
3-Aminopropyldimethylamine can be obtained by reacting dimethylamine with acrylonitrile via the intermediate product 3- (dimethylamino) propionitrile .
In 1994 around 15,000 t were produced worldwide.
properties
3-aminopropyldimethylamine is a slightly volatile, colorless to yellowish liquid with an amine-like odor, which is miscible with water. Your aqueous solution reacts strongly alkaline.
use
3-aminopropyldimethylamine is used as an intermediate in the manufacture of dyes and other chemical compounds, and as a corrosion inhibitor.
safety instructions
The vapors of 3-aminopropyldimethylamine can form an explosive mixture with air ( flash point 35 ° C, ignition temperature 215 ° C).
3-aminopropyldimethylamine is one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) has a data collection on possible hazards (" Screening Information Dataset “, SIDS) was made.
3-Aminopropyldimethylamine was included by the EU in 2014 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation in the Community's ongoing action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of 3-aminopropyldimethylamine were concerns about exposure of workers , high (aggregated) tonnage and widespread use as well as the suspected hazards from sensitizing properties. The re-evaluation has been running since 2014 and is carried out by Austria .
Individual evidence
- ↑ a b c d e f g h i j k l Entry on 3-aminopropyldimethylamine in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c Entry on N, N-DIMETHYL-1,3-PROPANEDIAMINE in the Hazardous Substances Data Bank , accessed on September 3, 2014.
- ↑ Data sheet 3- (Dimethylamino) -1-propylamine from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Entry on 3-aminopropyldimethylamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Toxicological assessment of dimethylaminopropionitrile (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ a b OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Propane, 1-Amino-3-dimethylamino- , accessed on October 3, 2014.
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 3-aminopropyldimethylamine , accessed on March 26, 2019.


