3-decanone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
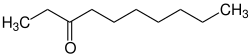
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-decanone | |||||||||||||||
| other names |
Ethyl heptyl ketone |
|||||||||||||||
| Molecular formula | C 10 H 20 O | |||||||||||||||
| Brief description |
light yellow liquid with a citrus orange, flowery and slightly greasy odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 156.27 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.825 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
−4 to −3 ° C |
|||||||||||||||
| boiling point |
204-205 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4240 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
3-decanone is a chemical compound from the ketone group .
Occurrence
3-decanone occurs naturally in bananas , mushrooms, lemon peel , mentha oil, heated butter, and cooked shrimp . It is a characteristic glandular product from males and females of Andrena praecox , Andrena helvola and females of Andrena clarkella as well as Manica bradleyi and Manica mutica .
Extraction and presentation
3-decanone, by a patented process by oxidation of alkanes with oxygen using a catalyst to be prepared under mild conditions. The preparation by palladium (II) -catalyzed Wacker oxidation of 1-decene in the presence of acid ( HClO 4 ) is also possible.
properties
3-decanone is a light yellow liquid with a citrus orange, floral and slightly greasy odor.
use
3-decanone can be used as a flavoring agent.
Individual evidence
- ↑ a b c d e f g h i data sheet 3-Decanon, ≥97%, FG at Sigma-Aldrich , accessed on April 25, 2017 ( PDF ).
- ↑ a b c d e f g h George A. Burdock: Fenaroli's Handbook of Flavor Ingredients, Sixth Edition . CRC Press, 2016, ISBN 978-1-4200-9086-4 , pp. 387 ( limited preview in Google Book search).
- ↑ Murry Blum: Chemical Defenses of Arthropods . Elsevier, 2012, ISBN 0-323-14555-8 , pp. 463 ( limited preview in Google Book search).
- ^ Bert Hölldobler, Edward O. Wilson: The Ants . Harvard University Press, 1990, ISBN 978-0-674-04075-5 , pp. 263 ( limited preview in Google Book search).
