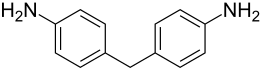
4,4'-diaminodiphenylmethane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4,4'-diaminodiphenylmethane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 14 N 2 | ||||||||||||||||||
| Brief description |
white to yellowish, flammable solid with an amine-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 198.27 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.1 g cm −3 |
||||||||||||||||||
| Melting point |
92 ° C |
||||||||||||||||||
| boiling point |
398 ° C |
||||||||||||||||||
| Vapor pressure |
approx. 0.00025 Pa (25 ° C) |
||||||||||||||||||
| solubility |
poor in water (1.25 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
of particular concern : carcinogenic ( CMR ); subject to approval |
||||||||||||||||||
| MAK |
Switzerland: 0.1 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4,4'-Diaminodiphenylmethane is a chemical compound from the group of diphenylmethane derivatives . Technical diaminodiphenylmethane consists of a mixture of isomeric compounds ( 2,2'-diaminodiphenylmethane , 2,4'-diaminodiphenylmethane ) and other amines .
Extraction and presentation
4,4'-Diaminodiphenylmethane is produced by a condensation reaction of formaldehyde with aniline in the presence of hydrochloric acid.
use
4,4'-Diaminodiphenylmethane is used as an intermediate in the production of synthetic resins (hardeners for epoxy resins), plastics, adhesives, dyes and vulcanization accelerators. Most of the amount produced (around 400,000 t per year in 1993) is used to produce 4,4'-diphenylmethane diisocyanate for the production of polyurethane (through reaction with phosgene ). After reductive cleavage of azo groups, the substance must not be released from textiles or leather products that come into direct contact with human skin for a longer period of time (Appendix 1 of the Consumer Goods Ordinance ).
safety instructions
4,4'-Diaminodiphenylmethane is classified as a carcinogen. The substance was added to the candidate list of substances of very high concern (SVHC) on October 28, 2008 .
See also
Individual evidence
- ↑ a b c d e f g Entry on 4,4′-diaminodiphenylmethane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Registration dossier on 4,4′-methylenedianiline ( Vapor pressure section ) at the European Chemicals Agency (ECHA), accessed on June 13, 2017.
- ↑ Entry on 4,4′-methylenedianiline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Entry in the SVHC list of the European Chemicals Agency , accessed on July 15, 2014.
- ↑ Entry in the register of substances subject to authorization of the European Chemicals Agency , accessed on July 15, 2014.
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limit values - current MAK and BAT values (search for 101-77-9 or 4,4′-diaminodiphenylmethane ), accessed on November 2, 2015.
- ↑ a b c European Union: Risk Assessment Report (Final Report November 2001) (PDF; 3.0 MB)
- ↑ Shau-Tarng Lee: Polymeric Foams. CRC Press, 2004, ISBN 978-0-203-50614-1 , p. 257 ( limited preview in Google book search).



