4,5-dibromopyrocatechol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4,5-dibromopyrocatechol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 Br 2 O 2 | ||||||||||||||||||
| Brief description |
white crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 267,90 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4,5-Dibromopyrocatechol is a chemical compound that belongs to both the phenols and the halogen aromatic compounds . It is isomeric to 3,5-dibromopyrocatechol and 3,6-dibromopyrocatechol .
presentation
4,5-Dibromopyrocatechol can be obtained from catechol by reacting it with elemental bromine in glacial acetic acid.
Reactions
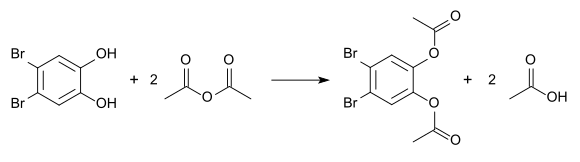
Esterification with acetic anhydride produces the diacetate, which has a melting point of 110 ° C.
The dimethyl ether ( 4,5-dibromoveratrole ), which is obtained by reacting with dimethyl sulfate , has a melting point of 92 to 93 ° C.
Further bromination with 1 mole of bromine in glacial acetic acid leads to 3,4,5-tribromopyrocatechol (CAS number: 2747-17-3), the melting point of which is 139–141 ° C. Complete bromination gives tetrabromo-catechol .
use
4,5-Dibromopyrocatechol is used as a starting material for the synthesis of 4,5-disubstituted 1,2-dibromobenzenes . It is also the starting material for the production of 4,5-diether-substituted phthalonitriles via a Rosenmund-von Braun reaction .
Individual evidence
- ^ A b c Moritz Kohn: "Bromination of Catechol", in: J. Am. Chem. Soc. , 1951 , 73 (1), pp. 480-480; doi : 10.1021 / ja01145a519
- ↑ a b c data sheet 4,5-dibromobenzene-1,2-diol from Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ^ A b J. Buckingham: Dictionary of organic compounds, Volume 9, p. 1914 ( limited preview in the Google book search).
- ↑ a b c K. M. Kadish, R. Guilard, KM Smith: "The porphyrin handbook", Vol. 11-20, p. 14 ( limited preview in the Google book search).
- ↑ D. Ximing, X. Huijun: "The synthesis and film-forming property of a new amphiphilic phthalocyanine" in: Dyes and Pigments , 1998 , 39 (4) , pp. 223-229; doi : 10.1016 / S0143-7208 (98) 00005-9 .