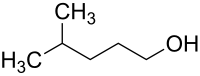
4-methyl-1-pentanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-methyl-1-pentanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 14 O | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 102.17 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.821 g cm −3 (25 ° C) |
|||||||||||||||
| boiling point |
160-165 ° C |
|||||||||||||||
| Refractive index |
1.414 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
4-methyl-1-pentanol is a chemical compound from the group of alkanols .
Occurrence
4-methyl-1-pentanol occurs naturally in tobacco . The connection has also been demonstrated in wine and longan fruit .
Extraction and presentation
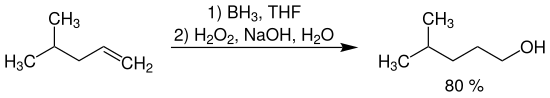
4-methyl-1-pentanol can be obtained by hydroboration of 4-methyl-1-pentene with diborane in THF as solvent (borane-THF complex) and subsequent oxidation with basic hydrogen peroxide solution.
Due to the pronounced regioselectivity of the hydroboration, the anti-Markovnikov alcohol is almost exclusively formed. The desired product is obtained in about 80% yield .
The compound can also be prepared by the reaction of isoamyl magnesium bromide with formaldehyde and other synthetic methods.
properties
4-methyl-1-pentanol is a colorless liquid.
Individual evidence
- ↑ a b c d e f g h i data sheet 4-methyl-1-pentanol, 97% from Sigma-Aldrich , accessed on January 14, 2019 ( PDF ).
- ↑ Alan Rodgman, Thomas A. Perfetti: The Chemical Components of Tobacco and Tobacco Smoke, Second Edition . CRC Press, 2013, ISBN 978-1-4665-1548-2 , pp. 2107 ( limited preview in Google Book search).
- ↑ Guo Cheng, Y. e. Liu, Tai-Xin Yue, Zhen-Wen Zhang: Comparison between aroma compounds in wines from four Vitis vinifera grape varieties grown in different shoot positions. In: Food Science and Technology. 35, 2015, p. 237, doi : 10.1590 / 1678-457X.6438 .
- ↑ ET Contis, C.-T. Ho, CJ Mussinan, TH Parliment, Fereidoon Shahidi, AM Spanier: Food Flavors: Formation, Analysis and Packaging Influences . Elsevier, 1998, ISBN 978-0-08-053183-0 , pp. 362 ( limited preview in Google Book search).
- ↑ a b K. Peter C. Vollhardt, Neil E. Schore, Katrin-M. Roy: Organic chemistry . Ed .: Holger Butenschön. 5th edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2011, ISBN 978-3-527-32754-6 , p. 572 ( limited preview in Google Book search).
- ^ Frank C. Whitmore: Organic Chemistry, Volume One Part I: Aliphatic Compounds Part II: Alicyclic Compounds . Courier Corporation, 2012, ISBN 978-0-486-60700-9 , pp. 125 ( limited preview in Google Book Search).