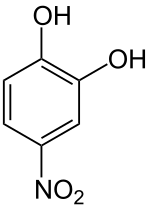
4-nitrocatechol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 4-nitrocatechol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 5 NO 4 | |||||||||||||||||||||
| Brief description |
yellow crystal fibers |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 155.11 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
174-176 ° C |
|||||||||||||||||||||
| solubility |
soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
4-nitrocatechol is a chemical compound that belongs to both phenols and nitroaromatics .
presentation
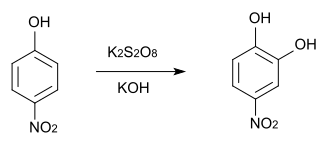
4-Nitro catechol can be obtained by reacting catechol with sulfuric acid and sodium nitrite . If an excess of nitrating agent is used, 3,4-dinitrocatechol is formed . A similar synthesis with potassium nitrate has also been reported.
When catechol reacts with fuming nitric acid in ether, a mixture of 3-nitrocatechol and 4-nitrocatechol is formed. This mixture can easily be separated by extraction with petroleum ether , since only the 3-nitrocatechol is soluble in petroleum ether.
Synthesis of 4-nitrophenol by Elbs oxidation is also possible.
properties
Physical Properties
4-Nitrocatechol is easily soluble in water, ethanol and ether , and sparingly soluble in benzene . In alkalis it dissolves with a purple color, the color change is very sensitive, so it can be used as an indicator .
Chemical properties
If barium hydroxide is added to an aqueous solution of 4-nitrocycatechol , a neutral barium salt is formed, which crystallizes in dark red flakes with three molecules of water . It gives off its water of crystallization at 130 ° C, the anhydrate is dark green in color. The reduction with tin and hydrochloric acid produces 4-aminocatechol .
Individual evidence
- ↑ a b Data sheet 4-nitrocatechol from AlfaAesar, accessed on February 19, 2010 ( PDF )(JavaScript required) .
- ↑ Data sheet 4-nitrocycatechol from Acros, accessed on February 19, 2010.
- ↑ a b Data sheet 4-Nitrocatechol from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ^ A b D. H. Rosenblatt, J. Epstein, M. Levitch: Some Nuclearly Substituted Catechols and their Acid Dissociation Constants , in: J. Am. Chem. Soc. , 1953 , 75 (13), pp. 3277-3278; doi : 10.1021 / ja01109a511 .
- ^ HE Roscoe, C. Schorlemmer: A treatise on chemistry , 1891; Full text .
- ↑ a b c d R. Benedikt: About the mononitro catechin . In: Reports of the German Chemical Society 1878 , 11 , pp. 362–363. Full text