9-borabicyclo (3.3.1) nonane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Crystal system |
monoclinic (dimer) |
||||||||||||||||||
| Space group |
C 2 / m (No. 12) (dimer) |
||||||||||||||||||
| Lattice parameters |
a = 1454 pm |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 9-borabicyclo [3.3.1] nonane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
150-152 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
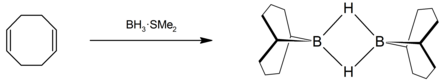
9-Borabicyclo [3.3.1] nonane , often abbreviated as 9-BBN , is an organoborane . The colorless solid is used in organic chemistry as a reagent for hydroboration .
presentation
9-BBN is produced by reacting 1,5-cyclooctadiene with borane complexes in ethereal solution (e.g. with tetrahydrofuran or dimethyl sulfide ).
properties
The compound is a dimer linked via the hydrogen atoms , which is easily cleaved in the presence of a reducible substrate. Like other organoboron compounds, 9-BBN is pyrophoric and is therefore mostly used as a solution in tetrahydrofuran .
As a solid, 9-BBN occurs exclusively as a dimer, also in certain organic solvents; however, there can also be an equilibrium between the dimeric and the (complexed with the solvent) monomeric form in solution.
use
In addition to hydroboration, 9-BBN can also be used in Suzuki reactions . Due to its high steric hindrance from the cyclooctyl substituent, 9-BBN leads almost exclusively to the anti-Markovnikov product in the hydroboration reaction compared to boranes .
Web links
Individual evidence
- ↑ a b c D. J. Brauer, C. Krüger: The Crystal and Molecular Structure of Bis-9-borabicyclo [3,3,1] nonane: a Study of the Boron-Carbon Bond . In: Acta Cryst. (1973), B29, pp. 1684-1690; doi : 10.1107 / S0567740873005261 .
- ↑ Data sheet 9-BBN (PDF) from Fisher Scientific , accessed February 13, 2014.
- ↑ a b Data sheet 9-Borabicyclo [3.3.1] nonane solution 0.5 M in THF from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ↑ John A. Soderquist, Herbert C. Brown : Simple, remarkably efficient route to high purity, crystalline 9-borabicyclo [3.3.1] nonane (9-BBN) dimer , J. Org. Chem. , 1981, 46, 22, Pp. 4599-4600, doi : 10.1021 / jo00335a067 .
- ↑ John A. Soderquist, Alvin Negron: 9-Borabicyclo [3.3.1] nonane, dimer In: Organic Syntheses . 70, 1992, p. 169, doi : 10.15227 / orgsyn.070.0169 ; Coll. Vol. 9, 1998, p. 95 ( PDF ).
- ^ HC Brown: Organic Syntheses via Boranes. John Wiley & Sons, Inc. New York 1975, ISBN 0-471-11280-1 .
- ↑ External identifiers or database links to 9-BBN (dimer) : CAS number: 21205-91-4, EC number: 629-228-4, ECHA InfoCard: 100.157.428 , PubChem : 16211404 , Wikidata : Q55961000 .
- ↑ Herbert C. Brown, Kung K. Wang, Charles G. Scouten: Hydroboration kinetics: Unusual kinetics for the reaction of 9-borabicyclo [3.3.1] nonane with representative alkenes ; Proc Natl Acad Sci USA, February 1980, 77 (2), pp. 698-702; Abstract .
- ↑ Tatsuo Ishiyama, Norio Miyaura, Akira Suzuki: Palladium (0) -catalyzed reaction of 9-alkyl-9-borabicyclo [3.3.1] nonane with 1-bromo-1-phenylthioethene: 4- (3-cyclohexenyl) -2- phenylthio-1-butene In: Organic Syntheses . 71, 1993, p. 89, doi : 10.15227 / orgsyn.071.0089 ; Coll. Vol. 9, 1998, p. 107 ( PDF ).