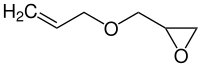
Allyl glycidyl ether
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without stereoisomerism | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Allyl glycidyl ether | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 10 O 2 | |||||||||||||||
| Brief description |
colorless liquid with a sweet, pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.97 g cm −3 |
|||||||||||||||
| Melting point |
−100 ° C |
|||||||||||||||
| boiling point |
154 ° C |
|||||||||||||||
| Vapor pressure |
2.6 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.433 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Allyl glycidyl ether is a chemical compound from the group of substituted ethers .
Extraction and presentation
Allyl glycidyl ether 3 can be obtained by condensation of allyl alcohol 1 and epichlorohydrin 2 and subsequent reaction with a base:
The technical product is in the form of a racemate .
properties
Allyl glycidyl ether is a colorless liquid with a sweet, pungent odor that is soluble in water. It decomposes when heated, producing carbon monoxide and carbon dioxide . The compound can polymerize on contact with acids and bases.
use
Allyl glycidyl ether can be used in hydrosilylation , including the hydrosilylation of siloxanes to epoxysiloxanes . It is also used as an additive in plastics and adhesives, as an intermediate in the production of other chemical compounds and as a stabilizer for polymers.
safety instructions
The vapors of allyl glycidyl ether can form an explosive mixture with air ( flash point 45 ° C). There is some evidence for the connection that indicates a possible mutagenic effect in humans.
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on allyl glycidyl ether in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c d e Michael Ash, Irene Ash: Handbook of Fillers, Extenders, and Diluents . Synapse Info Resources, 2007, ISBN 978-1-890595-96-8 , pp. 229 ( limited preview in Google Book search).
- ↑ a b Data sheet Allyl glycidyl ether, ≥99% from Sigma-Aldrich , accessed on December 31, 2016 ( PDF ).
- ↑ Entry on allyl glycidyl ether in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 31, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Clayton, GD and FE Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982, p. 2197.




