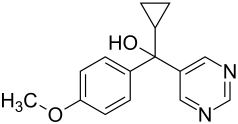
Ancymidol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ancymidol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 16 N 2 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 256.30 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
110-111 ° C |
||||||||||||||||||
| boiling point |
decomposes |
||||||||||||||||||
| solubility |
very sparingly soluble in water (650 mg l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ancymidol is a 1: 1 mixture of two enantiomeric chemical compounds from the group of pyrimidines . It is used as a growth regulator.
Extraction and presentation
Ancymidol can be obtained by reacting 4-methoxy-benzoylcyclopropane with 5-bromopyrimidine .
Admission
No pesticides containing this active ingredient are permitted in the EU or Switzerland .
Individual evidence
- ↑ a b c d e Entry on ancymidol in the GESTIS substance database of the IFA , accessed on March 21, 2014(JavaScript required) .
- ↑ Data sheet Ancymidol, plant cell culture tested, BioReagent at Sigma-Aldrich , accessed on March 21, 2014 ( PDF ).
- ↑ Ancymidol data sheet at Sigma-Aldrich , accessed on February 16, 2020 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 544 ( limited preview in Google Book search).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on ancymidol in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 17, 2016.